

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (7): 1125.doi: 10.7503/cjcu20160958
• Articles: Inorganic Chemistry • Previous Articles Next Articles
WANG Qiushuang, ZHENG Xiaoli, QU Xianglong, LI Rui, LI Xia*( )
)
Received:2016-12-29
Online:2017-07-10
Published:2017-05-02
Contact:
LI Xia
E-mail:xiali@cnu.edu.cn
Supported by:TrendMD:
WANG Qiushuang, ZHENG Xiaoli, QU Xianglong, LI Rui, LI Xia. Synthesis, Structure and Luminescence Property of Transition Metal Complexes with 1,3-Di(4-pyridyl)-propane and 1,2-Benzenedicarboxylic Acid†[J]. Chem. J. Chinese Universities, 2017, 38(7): 1125.
| Complex | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C42H40N4O10Co2 | C42H40N4O10Ni2 | C21H20N2O5Cd |
| Formula weight | 878.64 | 878.20 | 492.79 |
| Temperature/K | 120(2) | 120(2) | 120(2) |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21 | P21 | P21/c |
| a/nm | 1.16755(2) | 1.1609(3) | 1.04635(8) |
| b/nm | 1.4003(2) | 1.3986(3) | 1.01448(8) |
| c/nm | 1.18421(2) | 1.1847(3) | 1.87662(1) |
| β/(°) | 96.298(2) | 96.632(3) | 109.661(3) |
| V/nm3 | 1.9244(5) | 1.9107(7) | 1.8759(2) |
| Z | 2 | 2 | 4 |
| Dc/(g·cm-3) | 1.516 | 1.526 | 1.745 |
| Absorption coefficient/mm-1 | 0.928 | 1.052 | 1.202 |
| F(000) | 908 | 912 | 992 |
| Crystal size/mm | 0.38×0.34×0.30 | 0.42×0.15×0.10 | 0.15×0.10×0.07 |
| θ range for data collection/(°) | 1.730—25.249 | 2.26—25.25 | 2.07—27.63 |
| Limiting indices | -14≤h≤10, -16≤k≤16, | -13≤h≤13, -16≤k≤8, | -13≤h≤12, -13≤k≤13, |
| -12≤l≤14 | -14≤l≤13 | -24≤l≤20 | |
| Reflections collected | 9762 | 9035 | 10882 |
| Rint | 0.0220 | 0.0322 | 0.0311 |
| Data/restraints/parameters | 6637/7/535 | 4556/31/554 | 4307/39/280 |
| Goodness-of-fit on F2 | 1.028 | 1.062 | 1.025 |
| Final R indices[I>2σ(I)] | R1=0.0373, wR2=0.0887 | R1=0.0501, wR2= 0.1463 | R1=0.0284, wR2= 0.0604 |
| R indices(all data) | R1=0.0405, wR2=0.0907 | R1=0.0525, wR2=0.1478 | R1=0.0358, wR2= 0.0636 |
| CCDC No. | 1522654 | 1522656 | 1522655 |
Table 1 Crystallographic data of complexes 1—3
| Complex | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C42H40N4O10Co2 | C42H40N4O10Ni2 | C21H20N2O5Cd |
| Formula weight | 878.64 | 878.20 | 492.79 |
| Temperature/K | 120(2) | 120(2) | 120(2) |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21 | P21 | P21/c |
| a/nm | 1.16755(2) | 1.1609(3) | 1.04635(8) |
| b/nm | 1.4003(2) | 1.3986(3) | 1.01448(8) |
| c/nm | 1.18421(2) | 1.1847(3) | 1.87662(1) |
| β/(°) | 96.298(2) | 96.632(3) | 109.661(3) |
| V/nm3 | 1.9244(5) | 1.9107(7) | 1.8759(2) |
| Z | 2 | 2 | 4 |
| Dc/(g·cm-3) | 1.516 | 1.526 | 1.745 |
| Absorption coefficient/mm-1 | 0.928 | 1.052 | 1.202 |
| F(000) | 908 | 912 | 992 |
| Crystal size/mm | 0.38×0.34×0.30 | 0.42×0.15×0.10 | 0.15×0.10×0.07 |
| θ range for data collection/(°) | 1.730—25.249 | 2.26—25.25 | 2.07—27.63 |
| Limiting indices | -14≤h≤10, -16≤k≤16, | -13≤h≤13, -16≤k≤8, | -13≤h≤12, -13≤k≤13, |
| -12≤l≤14 | -14≤l≤13 | -24≤l≤20 | |
| Reflections collected | 9762 | 9035 | 10882 |
| Rint | 0.0220 | 0.0322 | 0.0311 |
| Data/restraints/parameters | 6637/7/535 | 4556/31/554 | 4307/39/280 |
| Goodness-of-fit on F2 | 1.028 | 1.062 | 1.025 |
| Final R indices[I>2σ(I)] | R1=0.0373, wR2=0.0887 | R1=0.0501, wR2= 0.1463 | R1=0.0284, wR2= 0.0604 |
| R indices(all data) | R1=0.0405, wR2=0.0907 | R1=0.0525, wR2=0.1478 | R1=0.0358, wR2= 0.0636 |
| CCDC No. | 1522654 | 1522656 | 1522655 |
| Co1—O6 | 0.2020(4) | Co1—O1 | 0.2153(4) | Co2—O8 | 0.2102(4) |
|---|---|---|---|---|---|
| Co1—N2A | 0.2105(5) | Co1—O2 | 0.2212(4) | Co2—N3 | 0.2112(5) |
| Co1—N1 | 0.2110(5) | Co2—O4B | 0.1987(4) | Co2—O10 | 0.2185(4) |
| Co1—O5 | 0.2152(4) | Co2—N4C | 0.2095(5) | Co2—O9 | 0.2237(4) |
| O6—Co1—N2A | 100.21(18) | O6—Co1—O2 | 94.71(16) | O8—Co2—N3 | 94.64(16) |
| O6—Co1—N1 | 92.04(17) | N2A—Co1—O2 | 164.30(16) | O4B—Co2—O10 | 89.72(16) |
| N2A—Co1—N1 | 92.14(18) | N1—Co1—O2 | 92.18(17) | N4C—Co2—O10 | 84.56(17) |
| O6—Co1—O5 | 90.58(16) | O5—Co1—O2 | 89.73(16) | O8—Co2—O10 | 85.50(15) |
| N2A—Co1—O5 | 85.30(17) | O1—Co1—O2 | 60.59(14) | N3—Co2—O10 | 179.62(18) |
| N1—Co1—O5 | 176.63(18) | O4B—Co2—N4C | 100.76(18) | O4B—Co2—O9 | 97.62(15) |
| O6—Co1—O1 | 154.37(16) | O4B—Co2—O8 | 158.35(16) | N4C—Co2—O9 | 159.14(16) |
| N2A—Co1—O1 | 103.95(17) | N4C—Co2—O8 | 99.79(17) | O8—Co2—O9 | 61.00(14) |
| N1—Co1—O1 | 95.45(17) | O4B—Co2—N3 | 90.28(17) | N3—Co2—O9 | 94.61(16) |
| O5—Co1—O1 | 83.07(15) | N4C—Co2—N3 | 95.07(18) | O10—Co2—O9 | 85.76(15) |
Table 2 Selected bond lengths(nm) and bond angles(°) for complex 1*
| Co1—O6 | 0.2020(4) | Co1—O1 | 0.2153(4) | Co2—O8 | 0.2102(4) |
|---|---|---|---|---|---|
| Co1—N2A | 0.2105(5) | Co1—O2 | 0.2212(4) | Co2—N3 | 0.2112(5) |
| Co1—N1 | 0.2110(5) | Co2—O4B | 0.1987(4) | Co2—O10 | 0.2185(4) |
| Co1—O5 | 0.2152(4) | Co2—N4C | 0.2095(5) | Co2—O9 | 0.2237(4) |
| O6—Co1—N2A | 100.21(18) | O6—Co1—O2 | 94.71(16) | O8—Co2—N3 | 94.64(16) |
| O6—Co1—N1 | 92.04(17) | N2A—Co1—O2 | 164.30(16) | O4B—Co2—O10 | 89.72(16) |
| N2A—Co1—N1 | 92.14(18) | N1—Co1—O2 | 92.18(17) | N4C—Co2—O10 | 84.56(17) |
| O6—Co1—O5 | 90.58(16) | O5—Co1—O2 | 89.73(16) | O8—Co2—O10 | 85.50(15) |
| N2A—Co1—O5 | 85.30(17) | O1—Co1—O2 | 60.59(14) | N3—Co2—O10 | 179.62(18) |
| N1—Co1—O5 | 176.63(18) | O4B—Co2—N4C | 100.76(18) | O4B—Co2—O9 | 97.62(15) |
| O6—Co1—O1 | 154.37(16) | O4B—Co2—O8 | 158.35(16) | N4C—Co2—O9 | 159.14(16) |
| N2A—Co1—O1 | 103.95(17) | N4C—Co2—O8 | 99.79(17) | O8—Co2—O9 | 61.00(14) |
| N1—Co1—O1 | 95.45(17) | O4B—Co2—N3 | 90.28(17) | N3—Co2—O9 | 94.61(16) |
| O5—Co1—O1 | 83.07(15) | N4C—Co2—N3 | 95.07(18) | O10—Co2—O9 | 85.76(15) |
| Ni1—O6 | 0.2009(6) | Ni1—O1 | 0.2128(5) | Ni2—N3 | 0.2075(6) |
|---|---|---|---|---|---|
| Ni1—N2A | 0.2053(7) | Ni1—O2 | 0.2145(5) | Ni2—O8 | 0.2080(5) |
| Ni1—N1 | 0.2066(6) | Ni2—O4B | 0.1985(5) | Ni2—O10 | 0.2117(5) |
| Ni1—O5 | 0.2108(6) | Ni2—N4C | 0.2048(7) | Ni2—O9 | 0.2178(5) |
| O6—Ni1—N2A | 98.3(2) | O6—Ni1—O2 | 96.6(2) | N3—Ni2—O8 | 93.5(2) |
| O6—Ni1—N1 | 91.6(2) | N2A—Ni1—O2 | 164.2(2) | O4B—Ni2—O10 | 89.8(2) |
| N2A—Ni1—N1 | 91.5(3) | N1—Ni1—O2 | 93.2(2) | N4C—Ni2—O10 | 85.0(2) |
| O6—Ni1—O5 | 90.5(2) | O5—Ni1—O2 | 88.8(2) | N3—Ni2—O10 | 179.6(3) |
| N2A—Ni1—O5 | 85.9(2) | O1—Ni1—O2 | 62.2(2) | O8—Ni2—O10 | 86.6(2) |
| N1—Ni1—O5 | 176.9(3) | O4B—Ni2—N4C | 97.6(2) | O4B—Ni2—O9 | 100.1(2) |
| O6—Ni1—O1 | 158.1(2) | O4B—Ni2—N3 | 90.3(2) | N4C—Ni2—O9 | 160.3(2) |
| N2A—Ni1—O1 | 102.4(2) | N4C—Ni2—N3 | 94.6(3) | N3—Ni2—O9 | 94.0(2) |
| N1—Ni1—O1 | 94.8(2) | O4B—Ni2—O8 | 162.1(2) | O8—Ni2—O9 | 62.2(2) |
| O5—Ni1—O1 | 84.0(2) | N4C—Ni2—O8 | 99.5(2) | O10—Ni2—O9 | 86.4(2) |
Table 3 Selected bond lengths(nm) and bond angles(°) for complex 2*
| Ni1—O6 | 0.2009(6) | Ni1—O1 | 0.2128(5) | Ni2—N3 | 0.2075(6) |
|---|---|---|---|---|---|
| Ni1—N2A | 0.2053(7) | Ni1—O2 | 0.2145(5) | Ni2—O8 | 0.2080(5) |
| Ni1—N1 | 0.2066(6) | Ni2—O4B | 0.1985(5) | Ni2—O10 | 0.2117(5) |
| Ni1—O5 | 0.2108(6) | Ni2—N4C | 0.2048(7) | Ni2—O9 | 0.2178(5) |
| O6—Ni1—N2A | 98.3(2) | O6—Ni1—O2 | 96.6(2) | N3—Ni2—O8 | 93.5(2) |
| O6—Ni1—N1 | 91.6(2) | N2A—Ni1—O2 | 164.2(2) | O4B—Ni2—O10 | 89.8(2) |
| N2A—Ni1—N1 | 91.5(3) | N1—Ni1—O2 | 93.2(2) | N4C—Ni2—O10 | 85.0(2) |
| O6—Ni1—O5 | 90.5(2) | O5—Ni1—O2 | 88.8(2) | N3—Ni2—O10 | 179.6(3) |
| N2A—Ni1—O5 | 85.9(2) | O1—Ni1—O2 | 62.2(2) | O8—Ni2—O10 | 86.6(2) |
| N1—Ni1—O5 | 176.9(3) | O4B—Ni2—N4C | 97.6(2) | O4B—Ni2—O9 | 100.1(2) |
| O6—Ni1—O1 | 158.1(2) | O4B—Ni2—N3 | 90.3(2) | N4C—Ni2—O9 | 160.3(2) |
| N2A—Ni1—O1 | 102.4(2) | N4C—Ni2—N3 | 94.6(3) | N3—Ni2—O9 | 94.0(2) |
| N1—Ni1—O1 | 94.8(2) | O4B—Ni2—O8 | 162.1(2) | O8—Ni2—O9 | 62.2(2) |
| O5—Ni1—O1 | 84.0(2) | N4C—Ni2—O8 | 99.5(2) | O10—Ni2—O9 | 86.4(2) |
| Cd1—N1 | 0.2331(2) | Cd1—O3 | 0.2352(7) | Cd1—O5 | 0.2448(2) |
|---|---|---|---|---|---|
| Cd1—N2A | 0.2351(2) | Cd1—O1B | 0.2421(2) | Cd1—O4 | 0.2467(1) |
| Cd1—O2B | 0.2352(4) | ||||
| N1—Cd1—N2A | 161.86(7) | N2A—Cd1—O1B | 85.12(7) | O1B—Cd1—O5 | 95.46(6) |
| N1—Cd1—O2B | 95.12(7) | O2B—Cd1—O1B | 54.97(6) | N1—Cd1—O4 | 87.61(7) |
| N2A—Cd1—O2B | 97.82(6) | O3—Cd1—O1B | 139.52(6) | N2A—Cd1—O4 | 90.69(7) |
| N1—Cd1—O3 | 101.52(7) | N1—Cd1—O5 | 82.41(7) | O2B—Cd1—O4 | 139.69(6) |
| N2A—Cd1—O3 | 92.11(7) | N2A—Cd1—O5 | 80.05(7) | O3—Cd1—O4 | 54.56(6) |
| O2B—Cd1—O3 | 85.67(6) | O2B—Cd1—O5 | 150.34(6) | O1B—Cd1—O4 | 165.32(6) |
| N1—Cd1—O1B | 92.00(7) | O3—Cd1—O5 | 123.87(6) | O5—Cd1—O4 | 69.93(6) |
Table 4 Selected bond lengths(nm) and bond angles(°) for complex 3*
| Cd1—N1 | 0.2331(2) | Cd1—O3 | 0.2352(7) | Cd1—O5 | 0.2448(2) |
|---|---|---|---|---|---|
| Cd1—N2A | 0.2351(2) | Cd1—O1B | 0.2421(2) | Cd1—O4 | 0.2467(1) |
| Cd1—O2B | 0.2352(4) | ||||
| N1—Cd1—N2A | 161.86(7) | N2A—Cd1—O1B | 85.12(7) | O1B—Cd1—O5 | 95.46(6) |
| N1—Cd1—O2B | 95.12(7) | O2B—Cd1—O1B | 54.97(6) | N1—Cd1—O4 | 87.61(7) |
| N2A—Cd1—O2B | 97.82(6) | O3—Cd1—O1B | 139.52(6) | N2A—Cd1—O4 | 90.69(7) |
| N1—Cd1—O3 | 101.52(7) | N1—Cd1—O5 | 82.41(7) | O2B—Cd1—O4 | 139.69(6) |
| N2A—Cd1—O3 | 92.11(7) | N2A—Cd1—O5 | 80.05(7) | O3—Cd1—O4 | 54.56(6) |
| O2B—Cd1—O3 | 85.67(6) | O2B—Cd1—O5 | 150.34(6) | O1B—Cd1—O4 | 165.32(6) |
| N1—Cd1—O1B | 92.00(7) | O3—Cd1—O5 | 123.87(6) | O5—Cd1—O4 | 69.93(6) |
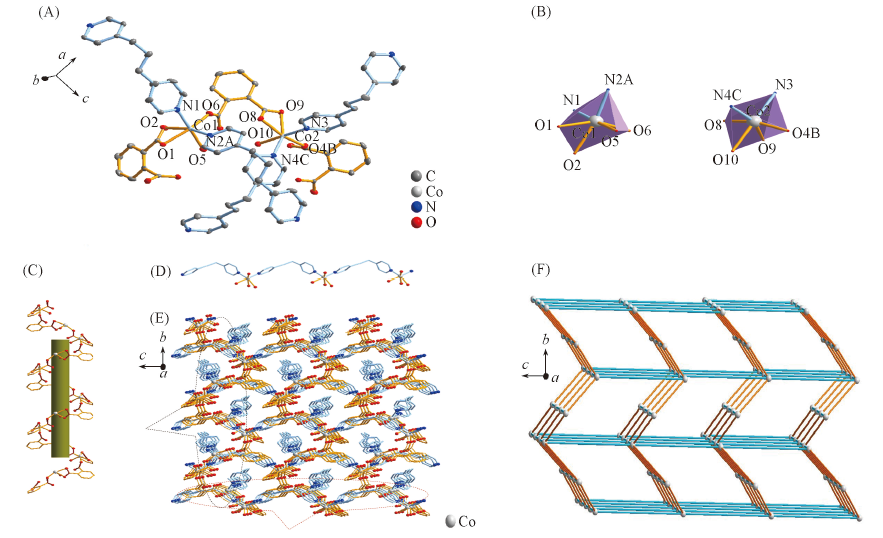
Fig.1 Asymmetric unit(A), coordination environment of Co(Ⅱ)(B), Co1-(1,2-bdc)-Co2 helix chain(C), Co2-(bpp)-Co2 chain(D), three-dimensional structure(E) and topological structure(F) of complex 1All hydrogen atoms in (A)—(E) are omitted; symmetry codes: A. x, y, z+1; B. -x, y-1/2, -z+2; C. x-1, y, z.
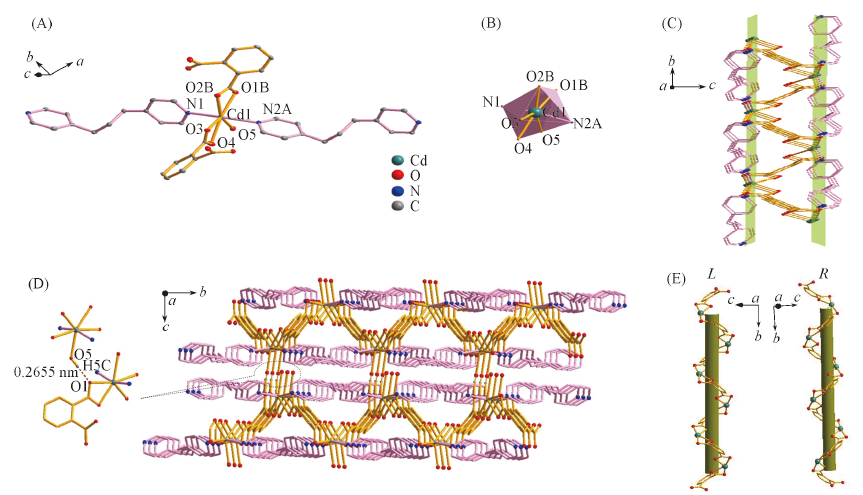
Fig.2 Asymmetric unit(A), coordination environment of Cd(Ⅱ)(B), two-dimensional network(C), three-dimensional structure by hydrogen bonds(D) and L-, R-helical chains(E) of complex 3All hydrogen atoms in (A)—(E) are omitted; symmetry codes: A. x+1, y-1, z; B. -x+2, y+1/2, -z+1/2; C. x, 1/2-y, -1/2+z.
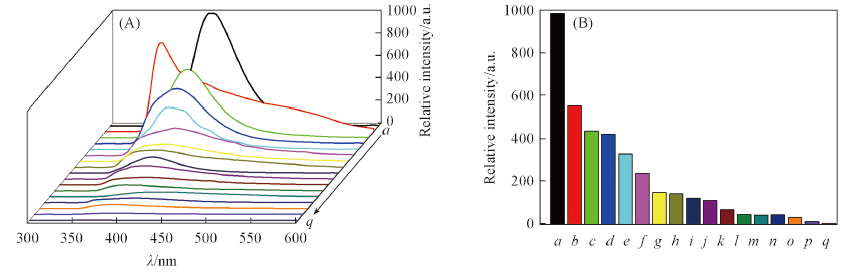
Fig.4 Emission spectra(A) and peak intensity at 408 nm(B) of complex 3 in different solvents Solvent: a. DMF; b. ethyl acetate; c. DMSO; d. dichloromethane; e. benzene; f. methanol; g. etanol; h. acetonitrile; i. hexane; j. formaldehyde; k. acetone; l. isopropanol; m. triethylamine; n. water; o. xylene; p. tetrahydrofuran; q. aniline.
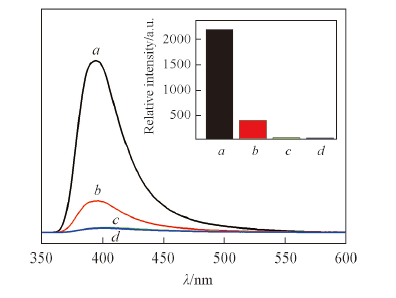
Fig.5 Emission spectra of complex 3 dispersed in ethanol solvent containing aniline with different concentrations(λex=314 nm)c(Aniline)/(mol·L-1): a. 0; b. 10-6; c. 10-5; d. 10-4. Inset shows peak intensity at 408 nm.
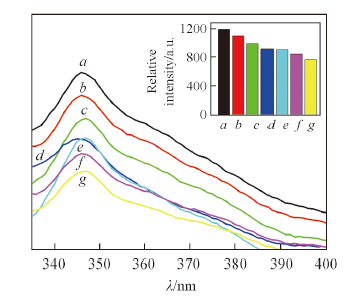
Fig.6 Emission spectra of complex 3 dispersed in ethanol solvent containing tetrahydrofuran with different concentrations(λex=314 nm)c(THF)/(mol·L-1): a. 0; b. 10-6; c. 10-5; d. 10-4; e. 10-3; f. 10-2; g. 10-1. Inset shows peak intensity at 345 nm.
| [1] | Furukawa H. K. N., Go Y. B., Aratani N., Choi S. B., Choi E., Yazaydin A. Ö., Snurr R. Q., O’Keeffe M., Kim J., Yaghi O. M., Science,2010, 329, 424—428 |
| [2] | Kreno L. E., Leong K., Farha O. K., Allendorf M., van Duyne R. P., Hupp J. T., Chem. Rev., 2012, 112, 1105—1125 |
| [3] | Nong R. Y., Kong J., Zhang J. H., Cheng L., Tang B., Xie S. M., Yuang L. M., Chem. J. Chinese Universities,2016, 37(1), 19—25 |
| (农蕊瑜, 孔娇, 章俊辉, 陈玲, 汤波, 谢生明, 袁黎明.高等学校化学学报, 2016, 37(1), 19—25) | |
| [4] | Brozek C. K., Dinca M., Chem. Soc. Rev., 2014, 43, 5456—5467 |
| [5] | Xu H., Chen R., Sun Q., La W., Su Q., Huang W., Liu X., Chem. Soc. Rev., 2014, 43, 3259—3302 |
| [6] | Hu Z. C., Deibert B. J., Li J., Chem. Soc. Rev., 2014, 43, 5815—5840 |
| [7] | Zhao L., Bai H. L., Sun E. J., Wang X. F., Wang Z. C., Chem. J. Chinese Universities,2016, 37(7), 1250—1256 |
| (赵仑, 白鹤龙, 孙二军, 王晓峰, 王子忱.高等学校化学学报, 2016, 37(7), 1250—1256) | |
| [8] | Pramanik S., Zheng C., Zhang X., Emge T. J., Li J., J. Am. Chem. Soc., 2011, 133, 4153—4155 |
| [9] | Vittal J. J., Coord. Chem. Rev., 2007, 251, 1781—1795 |
| [10] | Du M., Jiang X. J., Zhao X. J., Inorg. Chem., 2007, 46(10), 3984—3995 |
| [11] | Gang L. J., Wang L., Wang S. Y., Jing S. B., Chem. J. Chinese Universities,2016, 37(9), 1589—1595 |
| (高丽娟, 王莉,王圣燕,井淑波.高等学校化学学报, 2016, 37(9), 1589—1595) | |
| [12] | He W. W., Li S. L., Zang H. Y., Yang G. S., Zhang S. R., Su Z. M., Lan Y. Q., Coord. Chem. Rev., 2014, 279, 141—160 |
| [13] | Matsuda R., Kitaura R., Kitagawa S., Kubota Y., Belosludov R. V., Kobayashi T. C., Sakamoto H., Chiba T., Takat M.,Kawazoe Y.,Mit Y., Nature,2005, 436(7048), 238—241 |
| [14] | Debal K. S., Prakash M., Sudip K. M., Partha M., RSC Adv., 2015, 5, 102076—102084 |
| [15] | Xu H., Hu H. C., Cao C. S., Inorg. Chem., 2015, 54, 4585—4587 |
| [16] | Zheng T. T., Zhao J., Fang Z. W., Li M. T., Sun C. Y., Li X., Wang X. L., Su Z. M., Dalton Trans., 2017, 46, 2456—2461 |
| [17] | Seong Y. L., Kwon H. B., RSC Adv., 2017, 7, 290—299 |
| [18] | Chen S. G., Shi Z. Z., Qin L., Jia H. L., Cryst. Growth Des., 2017, 17, 67—72 |
| [19] | Hou S., Liu Q. K., Ma J. P., Dong Y. B., Inorg. Chem., 2013, 52, 3225—3235 |
| [20] | Xu X. Y., Yan B., ACS Appl. Mater. Interfaces,2015, 7, 721—729 |
| [21] | Lu Y., Yan B., Liu K. L., Chem. Commun., 2014, 50, 9969—9972 |
| [22] | Huang X. L., Liu L., Gao M. L., Han Z. B., RSC Adv., 2016, 6, 87945—87949 |
| [23] | Zhang X. F., Liu X. L., Lu R., Zhang H. J., Gong P., J. Mater. Chem., 2012, 22, 1167—1172 |
| [24] | Håkansson K., Coorey R. V., Zubarev R. A., Talrose V. L., Hakansson P., J. Mass Spectrom., 2000, 35, 337—346 |
| [25] | del Nogal S. M., Sappo C. P., Perez P. J. L., Cordero B. M., Anal. Bioanal. Chem. , 2012, 404,2007—2015 |
| [26] | Imasaka T., Tashiro K., Ishibashi N., Anal. Chem., 1986, 58, 3242—3244 |
| [27] | Hou Y. L., Xu H., Cheng R. R., Zhao B., Chem. Commun., 2015, 51, 6769—6772 |
| [28] | Chen X. L., Li Z. B., Zhu Y. X., Xu J. G., Anal. Chimica Acta,2004, 505, 283—287 |
| [29] | Fan T. T., Li J. J., Qu X. L., Cryst. Eng. Comm., 2015, 17, 9443—9451 |
| [30] | Lu W. G., Wei Z. W., Gu Z. Y., Liu T. F., Park J. H., Tian J., Zhang M. W., Zhang Q., Gentle I., Thomas., Bosch M., Zhou H. C., Chem. Soc. Rev., 2014, 43, 5561—5593 |
| [31] | Sheldrick G.M., SHELXS 97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, 1997 |
| [32] | Sheldrick G M., SHELXL 97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen, 1997 |
| [33] | Zhang L. P., Ma J. F., Yang J., Pang Y. Y., Ma J., Inorg. Chem., 2010, 49, 1535—1550 |
| [34] | Guo J., Ma J. F., Liu B., Kan W. Q., Yang J., Cryst. Growth Des., 2011, 11, 3609—3621 |
| [35] | Zhao J., Wang X. L., Shi X., Inorg. Chem., 2011, 50, 3198—3205 |
| [36] | Majumder S., Mandal L., Mohanta S., Inorg. Chem., 2012, 51, 8739—8749 |
| [37] | Gu J. Z., Liang X. X., Cui Y. H., Wu J., Shi Z. F., Cryst. Eng. Comm., 2017, 19(18), 2570—2588 |
| [38] | Zhu X. D., Zhou W. X., Liu R. M., Ding Y. J., Lu J., Proserpio D. M., Cryst. Eng. Comm., 2016, 18(24), 4530—4537 |
| [39] | Yang L. L., Xiao Q. C., Shao K. Z., Su Z. M., Cryst. Eng. Comm., 2016, 18(25), 4765—4771 |
| [40] | Zhang X., Wang Z. J., Chen S. G., Shi Z. Z., Chen J. X., Zheng H. G., Dalton Trans., 2017, 46(7), 2332—2338 |
| [41] | de Silva A. P., Gunaratne H. Q. N., Gunnlaugsson T., Huxley A. J. M., McCoy C. P., Rademacher J. T., Rice T. E., Chem. Rev., 1997, 97, 1515—1566 |
| [1] | GE Yicong, NIE Wanli, SUN Guofeng, CHEN Jiaxuan, TIAN Chong. Silver-catalyzed [5+1] Cyclization of 2-Vinylanilines with Benzisoxazoles [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220142. |
| [2] | LIU Qingqing, WANG Pu, WANG Yongshuai, ZHAO Man, DONG Huanli. Synthesis and Topochemical Polymerization Study of Naphthalene/perylene Imides Substituted Diacetylene Derivatives [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220091. |
| [3] | SHI Naike, ZHANG Ya, SANSON Andrea, WANG Lei, CHEN Jun. Uniaxial Negative Thermal Expansion and Mechanism in Zn(NCN) [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220124. |
| [4] | WANG Junyang, LIU Zheng, ZHANG Qian, SUN Chunyan, LI Hongxia. Application of DNA Silver Nanoclusters in the Fluorescence Biosensors based on Functional Nucleic Acids [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220010. |
| [5] | LU Cong, LI Zhenhua, LIU Jinlu, HUA Jia, LI Guanghua, SHI Zhan, FENG Shouhua. Synthesis, Structure and Fluorescence Detection Properties of a New Lanthanide Metal-Organic Framework Material [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220037. |
| [6] | WANG Mingzhi, ZHENG Yanping, WENG Weizheng. Catalytic Methane Combustion over CeO2 Supported PdO and Ce1‒x Pd x O2‒δ Species [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210816. |
| [7] | LI Qiao, ZHAO Yang, WANG Enju. Moisture Absorption Reaction and Fluorescence Property of Highly Active Michael System Based on Arylidenemalononitrile [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210690. |
| [8] | GAO Jing, HE Wentao, WANG Xinxin, XIANG Yushu, LONG Lijuan, QIN Shuhao. Preparation of DOPO Derivative Modified Carbon Nanotubes and Their Effect on Flame Retardancy of Polylactic Acid [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210670. |
| [9] | LI Xiaohui, WEI Aijia, MU Jinping, HE Rui, ZHANG Lihui, WANG Jun, LIU Zhenfa. Effects of SmPO4 Coatingon Electrochemical Performance of High-voltage LiNi0.5Mn1.5O4 Cathode Materials [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210546. |
| [10] | TIAN Xueqin, MO Zheng, DING Xin, WU Pengyan, WANG Yu, WANG Jian. A Squaramide-containing Luminescent Metal-organic Framework as a High Selective Sensor for Histidine [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210589. |
| [11] | ZHANG Liling, LIU Liu, ZHENG Mingqiu, FANG Wenkai, LIU Da, TANG Hongwu. Dual Signal Detection of HPV16 DNA by CRISPR/Cas12a Biosensing System Based on Upconversion Luminescent Resonance Energy Transfer [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220412. |
| [12] | WU Zexin, ZHU Yuanjie, WANG Hongzhong, WANG Junan, HE Ying. Methyl-modified Carbazole/Diphenyl Sulfone-based AIE-TADF Blue Emitter and Its OLEDs [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220371. |
| [13] | LI Dan, XIAO Liping, FAN Jie. Inorganic-based Surface Materials with Anti-SARS-CoV-2 Properties and Their Mechanisms of Action [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220301. |
| [14] | LIU Miao, LIU Ruibo, LIU Badi, QIAN Ying. Synthesis, Two-photon Fluorescence Imaging and Photodynamic Therapy of Lysosome-targeted Indole-BODIPY Photosensitizer [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220326. |
| [15] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||