

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (7): 1380.doi: 10.7503/cjcu20150995
• Physical Chemistry • Previous Articles Next Articles
ZHANG Guoqiang, ZHENG Huayan, HAO Zhiqiang, LI Zhong*( )
)
Received:2015-12-30
Online:2016-07-10
Published:2016-06-16
Contact:
LI Zhong
E-mail:lizhong@tyut.edu.cn
Supported by:CLC Number:
TrendMD:
ZHANG Guoqiang, ZHENG Huayan, HAO Zhiqiang, LI Zhong. Effect of Activated Carbon Surface Chemistry on the Properties of Cu Particles and the Catalytic Performance for Oxidative Carbonylation of Methanol†[J]. Chem. J. Chinese Universities, 2016, 37(7): 1380.
| Sample | SBET/(m2·g-1) | VP/(cm3·g-1) | DP/nm | Smicro/(m2·g-1) |
|---|---|---|---|---|
| AC | 1597 | 0.80 | 2.00 | 1514 |
| AC-N2-600 | 1436 | 0.71 | 1.98 | 1364 |
| AC-N2-800 | 1480 | 0.73 | 1.98 | 1407 |
| AC-NH3-600 | 1442 | 0.72 | 2.01 | 1376 |
| AC-NH3-800 | 1465 | 0.73 | 2.00 | 1389 |
| Cu/AC | 1275 | 0.65 | 1.98 | 1190 |
| Cu/AC-N2-600 | 1227 | 0.61 | 1.98 | 1163 |
| Cu/AC-N2-800 | 1256 | 0.64 | 2.00 | 1184 |
| Cu/AC-NH3-600 | 1284 | 0.64 | 2.00 | 1195 |
| Cu/AC-NH3-800 | 1293 | 0.67 | 1.98 | 1201 |
Table 1 Textual property of the AC supports and Cu/AC catalysts
| Sample | SBET/(m2·g-1) | VP/(cm3·g-1) | DP/nm | Smicro/(m2·g-1) |
|---|---|---|---|---|
| AC | 1597 | 0.80 | 2.00 | 1514 |
| AC-N2-600 | 1436 | 0.71 | 1.98 | 1364 |
| AC-N2-800 | 1480 | 0.73 | 1.98 | 1407 |
| AC-NH3-600 | 1442 | 0.72 | 2.01 | 1376 |
| AC-NH3-800 | 1465 | 0.73 | 2.00 | 1389 |
| Cu/AC | 1275 | 0.65 | 1.98 | 1190 |
| Cu/AC-N2-600 | 1227 | 0.61 | 1.98 | 1163 |
| Cu/AC-N2-800 | 1256 | 0.64 | 2.00 | 1184 |
| Cu/AC-NH3-600 | 1284 | 0.64 | 2.00 | 1195 |
| Cu/AC-NH3-800 | 1293 | 0.67 | 1.98 | 1201 |
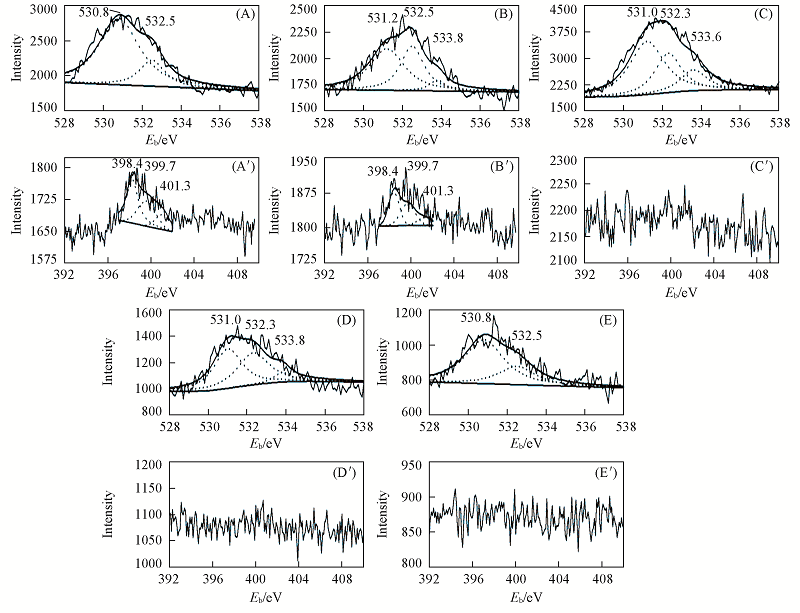
Fig.1 XPS spectra of O1s(A—E) and N1s(A'—E') of the AC supports(A, A') AC-NH3-800; (B, B') AC-NH3-600; (C, C') AC; (D, D') AC-N2-600; (E, E') AC-N2-800.
| Sample | Elemental content(mass fraction, %) | ||
|---|---|---|---|
| C | O | N | |
| AC | 91.1 | 7.9 | |
| AC-N2-600 | 94.5 | 5.5 | |
| AC-N2-800 | 95.6 | 4.6 | |
| AC-NH3-600 | 96.5 | 2.7 | 0.8 |
| AC-NH3-800 | 97.0 | 1.6 | 1.4 |
Table 2 Carbon, oxygen and nitrogen contents of the AC samples obtained by XPS
| Sample | Elemental content(mass fraction, %) | ||
|---|---|---|---|
| C | O | N | |
| AC | 91.1 | 7.9 | |
| AC-N2-600 | 94.5 | 5.5 | |
| AC-N2-800 | 95.6 | 4.6 | |
| AC-NH3-600 | 96.5 | 2.7 | 0.8 |
| AC-NH3-800 | 97.0 | 1.6 | 1.4 |
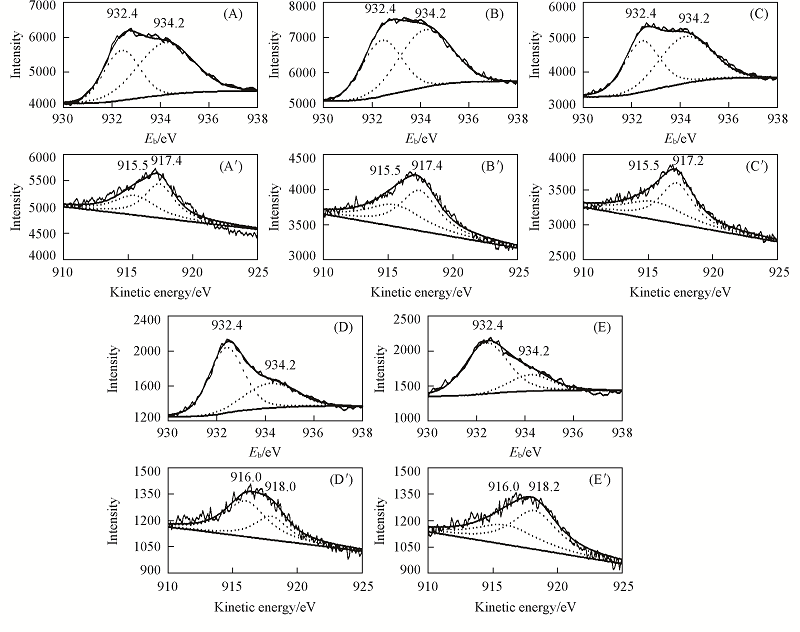
Fig.2 Cu2p32 XPS(A—E) and Cu LMM Auger spectra(A'—E') of the Cu/AC catalysts(A, A') AC-NH3-800; (B, B') AC-NH3-600; (C, C') AC; (D, D') AC-N2-600; (E, E') AC-N2-800.
| Catalyst | Eb of C | Kinetic energy of CuLMM/eV | Area of C | Area of CuLMMb(%) | C (%) | C (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu2+ | Cu++Cu0 | Cu+ | Cu0 | Cu2+ | Cu++Cu0 | Cu+ | Cu0 | |||
| Cu/AC-NH3-800 | 934.2 | 932.4 | 915.5 | 917.4 | 63.7 | 36.3 | 43.8 | 56.2 | 16.0 | 20.3 |
| Cu/AC-NH3-600 | 934.2 | 932.4 | 915.5 | 917.4 | 57.2 | 42.8 | 44.0 | 56.0 | 18.8 | 24.0 |
| Cu/AC | 934.2 | 932.4 | 915.5 | 917.2 | 53.9 | 46.1 | 44.4 | 55.6 | 20.5 | 25.6 |
| Cu/AC-N2-600 | 934.2 | 932.4 | 916.0 | 918.0 | 33.3 | 66.7 | 65.3 | 34.7 | 43.5 | 23.2 |
| Cu/AC-N2-800 | 934.2 | 932.4 | 916.0 | 918.2 | 21.5 | 78.5 | 40.6 | 59.4 | 31.8 | 46.7 |
Table 3 Cu2p32 XPS and CuLMM AES curve-fitting analysis of catalysts
| Catalyst | Eb of C | Kinetic energy of CuLMM/eV | Area of C | Area of CuLMMb(%) | C (%) | C (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu2+ | Cu++Cu0 | Cu+ | Cu0 | Cu2+ | Cu++Cu0 | Cu+ | Cu0 | |||
| Cu/AC-NH3-800 | 934.2 | 932.4 | 915.5 | 917.4 | 63.7 | 36.3 | 43.8 | 56.2 | 16.0 | 20.3 |
| Cu/AC-NH3-600 | 934.2 | 932.4 | 915.5 | 917.4 | 57.2 | 42.8 | 44.0 | 56.0 | 18.8 | 24.0 |
| Cu/AC | 934.2 | 932.4 | 915.5 | 917.2 | 53.9 | 46.1 | 44.4 | 55.6 | 20.5 | 25.6 |
| Cu/AC-N2-600 | 934.2 | 932.4 | 916.0 | 918.0 | 33.3 | 66.7 | 65.3 | 34.7 | 43.5 | 23.2 |
| Cu/AC-N2-800 | 934.2 | 932.4 | 916.0 | 918.2 | 21.5 | 78.5 | 40.6 | 59.4 | 31.8 | 46.7 |
| [1] | Fu Z. H., Ono Y., J. Mol. Catal. A,1997, 118(3), 293—299 |
| [2] | Ono Y., Appl. Catal. A, 1997, 155(2), 133—166 |
| [3] | Wang Y., Zhao X., Yuan B., Zhang B., Cong J., Appl. Catal. A,1998, 171(2), 255—260 |
| [4] | Li Z., Wang R., Zheng H., Xie K., Fuel,2010, 89(7), 1339—1343 |
| [5] | Ren J., Liu S., Li Z., Xie K., Catal. Commun., 2011, 12(5), 357—361 |
| [6] | Li Z., Fu T. J., Wang R. Y., Niu Y. Y., Zheng H. Y., Chem. J. Chinese Universities,2011, 32(6), 1366—1372(李忠, 付廷俊, 王瑞玉, 牛燕燕, 郑华艳. 高等学校化学学报, 2011, 32(6), 1366—1372) |
| [7] | Li Z., Zhu Q. F., Wang R. Y., Niu Y. Y., Zheng H. Y., Chin. J. Inorg. Chem., 2011, 27(4), 718—724(李忠, 朱琼芳, 王瑞玉, 牛燕燕, 郑华艳. 无机化学学报, 2011, 27(4), 718—724) |
| [8] | Ma X., Li Z., Wang B., Xu G., React. Kinet. Catal. Lett., 2002, 76(1), 179—187 |
| [9] | Jiang R., Wang S., Zhao X., Wang Y., Zhang C., Appl. Catal. A,2003, 238(1), 131—139 |
| [10] | Li Z., Wen C. M., Wang R. Y., Zheng H. Y., Xie K. C., Chem. J. Chinese Universities,2009, 30(10), 2024—2031(李忠, 文春梅, 王瑞玉, 郑华艳, 谢克昌. 高等学校化学学报, 2009, 30(10), 2024—2031) |
| [11] | Yan B., Huang S. Y., Wang S. P., Ma X. B., ChemCatChem,2014, 6(9), 2671—2679 |
| [12] | Ren J., Ren M., Wang D., Lin J., Li Z., J. Therm. Anal. Calorim., 2015, 120(3), 1929—1939 |
| [13] | Ren J., Wang W., Wang D., Zuo Z., Lin J., Li Z., Appl. Catal. A,2014, 472(0), 47—52 |
| [14] | Sun S., Li C., Zhang D., Wang Y., Appl. Surf. Sci., 2015, 333, 229—234 |
| [15] | Sun S., Wang Y., Yang Q., Appl. Surf. Sci., 2014, 313, 777—783 |
| [16] | Engeldinger J., Domke C., Richter M., Bentrup U., Appl. Catal. A,2010, 382(2), 303—311 |
| [17] | Engeldinger J., Richter M., Bentrup U., Phys. Chem. Chem.Phys., 2012, 14, 2183—2191 |
| [18] | Li Z., Wen C. M., Zheng H. Y., Xie K. C., Chem. J. Chinese Universities,2010, 31(1), 145—152(李忠, 文春梅, 郑华艳, 谢克昌. 高等学校化学学报, 2010, 31(1), 145—152) |
| [19] | Prado-Burguete C., Linares-Solano A., Rodríguez-Reinoso F., de Lecea C. S. M., J. Catal., 1989, 115(1), 98—106 |
| [20] | Zhang G., Li Z., Zheng H., Fu T., Ju Y., Wang Y., Appl. Catal. B,2015, 179(0), 95—105 |
| [21] | Rodrigues E. G., Pereira M. F. R., Chen X., Delgado J. J., Órfâo J. J. M., J. Catal., 2011, 281(1), 119—127 |
| [22] | Wang X., Li N., Webb J. A., Pfefferle L. D., Haller G. L., Appl. Catal. B,2010, 101(1/2), 21—30 |
| [23] | Xu J., Zhao J., Xu J., Zhang T., Li X., Di X., Ni J., Wang J., Cen J., Ind. Eng. Chem. Res., 2014, 53(37), 14272—14281 |
| [24] | Choi C. H., Park S. H., Chung M. W., Woo S. I., Carbon,2013, 55(0), 98—107 |
| [25] | Wu G., Swaidan R., Li D., Li N., Electrochim. Acta,2008, 53(26), 7622—7629 |
| [26] | Yang Y., Jia L., Hou B., Li D., Wang J., Sun Y., ChemCatChem., 2014, 6(1), 319—327 |
| [27] | Chizari K., Janowska I., Houllé M., Florea I., Ersen O., Romero T., Bernhardt P., Ledoux M. J., Pham-Huu C., Appl. Catal. A,2010, 380(1/2), 72—80 |
| [28] | Priyanka Subbaramaiah V., Srivastava V. C., Mall I. D., Sep. Purif. Technol., 2014, 125, 284—290 |
| [29] | Rodrigues E. G., Delgado J. J., Chen X., Pereira M. F. R., Órfâo J.J. M., Ind. Eng. Chem. Res., 2012, 51(49), 15884—15894 |
| [30] | Xiong B., Zhou Y., Zhao Y., Wang J., Chen X., O’Hayre R., Shao Z., Carbon,2013, 52(0), 181—192 |
| [31] | Horikawa T., Sakao N., Sekida T., Hayashi J. I., Do D. D., Katoh M., Carbon,2012, 50(5), 1833—1842 |
| [32] | Figueiredo J. L., Pereira M. F. R., Freitas M. M. A., Órfâo J. J. M., Carbon,1999, 37(9), 1379—1389 |
| [33] | Espinós J. P., Morales J., Barranco A., Caballero A., Holgado J. P., González-Elipe A. R., J. Phys. Chem. B,2002, 106(27), 6921—6929 |
| [34] | Teo J. J., Chang Y., Zeng H. C., Langmuir,2006, 22(17), 7369—7377 |
| [35] | Wang W. Z., Wang G. H., Wang X. S., Zhan Y. J., Liu Y. K., Zheng C. L., Adv. Mat., 2002, 14(1), 67—69 |
| [36] | Raimondi F., Geissler K., Wambach J., Wokaun A., Appl. Surf. Sci., 2002, 189(1/2), 59—71 |
| [37] | Zhao S., Yue H., Zhao Y., Wang B., Geng Y., Lv J., Wang S., Gong J., Ma X., J. Catal., 2013, 297(0), 142—150 |
| [38] | Coloma F., Sepúlveda-Escribano A., Rodríguez-Reinoso F., Appl. Catal. A,1995, 123(1), L1—L5 |
| [39] | Dandekar A., Baker R. T. K., Vannice M. A., J. Catal., 1999, 183(1), 131—154 |
| [40] | Guo Y., He J., Wang T., Xue H., Hu Y., Li G., Tang J., Sun X., J. Power. Sources,2011, 196(22), 9299—9307 |
| [41] | Li Y. H., Hung T. H., Chen C. W., Carbon,2009, 47(3), 850—855 |
| [42] | Hornés A., Bera P., Cámara A.L., Gamarra D., Munuera G., Martínez-Arias A., J. Catal., 2009, 268(2), 367—375 |
| [43] | Kefirov R., Penkova A., Hadjiivanov K., Dzwigaj S., Che M., Micropor. Mesopor. Mater., 2008, 116(1—3), 180—187 |
| [44] | Wang S. P., Li W., Dong Y. Y., Zhao Y. J., Ma X. B., Catal. Commun., 2015, 72, 43—48 |
| [1] | HUANG Mingxin, ZHOU Lei, WANG Xuezhong. Measurement of Particle Size Distribution of Battery Slurries Using Ultrasonic Attenuation Spectroscopy [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220040. |
| [2] | DOU Shuzhen, WANG Zhongshun, LYU Nan. Improving the Detection Performance of Surface-assisted Laser Desorption/ionization Mass Spectrometry by Silicon Nanostructures [J]. Chem. J. Chinese Universities, 2021, 42(4): 1156. |
| [3] | DONG Le, HUANG Xingliang, REN Junjie, DAI Xiaoping, LIU Zongyan, TIAN Hongfeng, WANG Zhidong, WU Xiaotong. Influence Mechanism of Particle Size and Distribution of Silica Sol in the Synthesis of Ferrierite Zeolite with High SiO2/Al2O3 Ratio [J]. Chem. J. Chinese Universities, 2020, 41(11): 2449. |
| [4] | YIN Jiao, ZHANG Guoqiang, YAN Lifei, JIA Dongsen, ZHENG Huayan, LI Zhong. Influence of Structure Evolution of CuY Catalyst During the Reaction Process on Its Catalytic Performance for Oxidative Carbonylation of Methanol† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1510. |
| [5] | XIAO Biyuan,QIU Jiangyuan,QIN Fanghong,WAN Ting,XU Yaqun,NONG Xiaohui,HUANG Zaiyin. Study on Particle Size Effect on Adsorption Thermodynamics and Kinetics of Cubic Nano-Cu2O † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2214. |
| [6] | FENG Jianguo, YANG Guantian, YUAN Xiaoyong, CHEN Qicheng, SUN Chencheng, YUAN Shuzhong. Preparation, Characterization and Release Properties of β-Cypermethrin Microcapsules† [J]. Chem. J. Chinese Universities, 2017, 38(11): 1974. |
| [7] | HUANG Zhaoliang, GAO Fangyuan, WANG Boliang, ZHANG Weibing. Simulation of the Formation and Fission of Charged Droplets in Electrospray Ion Source† [J]. Chem. J. Chinese Universities, 2016, 37(4): 633. |
| [8] | WANG Yuchun, ZHENG Huayan, LIU Bin, ZHANG Guoqiang, LI Zhong. Chloride-free CuY Catalyst Prepared by Solid State Reaction for Oxidative Carbonylation of Methanol† — Effect of Solid State Reactive Temperature and Copper Loading [J]. Chem. J. Chinese Universities, 2015, 36(12): 2540. |
| [9] | SUN Haijie, CHEN Lingxia, HUANG Zhenxu, LIU Shouchang, LIU Zhongyi. Particle Size Effect of Ru-Zn Catalysts on Selective Hydrogenation of Benzene to Cyclohexene† [J]. Chem. J. Chinese Universities, 2015, 36(10): 1969. |
| [10] | ZHANG Yanqing, ZHENG Huayan, ZHANG Riguang, LI Zhong, WANG Baojun, ZHAO Qiuyong. Density Functional Theory Investigation on the Effect of Alkali Metal Cations on the Catalytic Performance for Cu+Y Zeolites in Oxidative Carbonylation of Methanol† [J]. Chem. J. Chinese Universities, 2015, 36(10): 1945. |
| [11] | ZHENG Huayan, ZHANG Riguang, LI Zhong. Theoretical Studies on the Interaction of CO and CH3O on CuCl(111) Surface for Methanol Oxidative Carbonylation† [J]. Chem. J. Chinese Universities, 2014, 35(9): 1926. |
| [12] | WANG Yanen, CAO Shuang, WU Weihong, WU Min, TANG Yawen, LU Tianhong. Effect of Particle Size on the Electrocatalytic Activity of Pt/C Catalysts for Oxidation of Formic Acid† [J]. Chem. J. Chinese Universities, 2014, 35(11): 2455. |
| [13] | SUN Mei-Yu, PANG Xiu-Jiang, MA Xiu-Ming, HOU Wan-Guo. Preparation and Particle Size Controllability of Mg-Al Layered Double Hydroxides via Coprecipitation Method Using T-type Microchannel Reactor [J]. Chem. J. Chinese Universities, 2013, 34(7): 1691. |
| [14] | ZHENG Chu-Guang, ZHAO Hai-Bo. Monte Carlo Solution of Particle Size Distribution under Simultaneous Coagulation and Nucleation [J]. Chem. J. Chinese Universities, 2005, 26(11): 2086. |
| [15] | LIU Bing-Bing, YU Miao, LI Dong-Mei, CUI Tian, WANG Lin, YAO Ming-Guang, YU Shi-Dan, ZOU Guang-Tian, WANG Xu, ZHAO Bing, SUNDQVIST Bertil . Effects of Silver Films with Different Nano-particle Sizes on SERS of Single-walled Carbon Nanotubes [J]. Chem. J. Chinese Universities, 2005, 26(10): 1930. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||