

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (12): 2540.doi: 10.7503/cjcu20150285
• Physical Chemistry • Previous Articles Next Articles
WANG Yuchun1,2, ZHENG Huayan1, LIU Bin1, ZHANG Guoqiang1, LI Zhong1,*( )
)
Received:2015-04-13
Online:2015-12-10
Published:2015-10-12
Contact:
LI Zhong
E-mail:lizhong@tyut.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Yuchun, ZHENG Huayan, LIU Bin, ZHANG Guoqiang, LI Zhong. Chloride-free CuY Catalyst Prepared by Solid State Reaction for Oxidative Carbonylation of Methanol† — Effect of Solid State Reactive Temperature and Copper Loading[J]. Chem. J. Chinese Universities, 2015, 36(12): 2540.
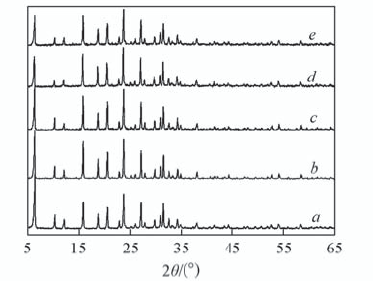
Fig.1 XRD patterns of NH4Y and catalysts 10CuY with different temperature of solid state reaction(SSR) a. NH4Y; b. 10CuY170; c.10CuY200; d. 10CuY250;e. 10CuY280.
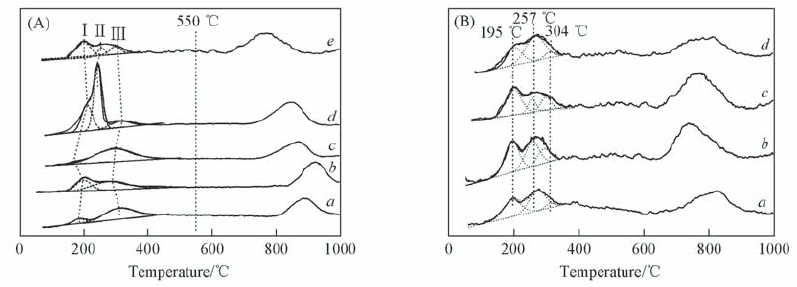
Fig.2 H2-TPR patterns and Gaussian fitting of low temperature peaks (A) a. Cu2+-Y(6.3); b. CuY(6.3); c. 6CuY250; d. CuY(10.6); e. 10CuY250.(B) a. 10CuY170; b. 10CuY200; c. 10CuY250; d. 10CuY280.
| Catalyst | Mass fraction(%) | ||||
|---|---|---|---|---|---|
| Cu2+(Supercage) | Cu2+(Sodalite cage) | CuO | Cu+ | Cusum | |
| 10CuY170 | 2.99 | 0.84 | 2.36 | 3.30 | 9.49 |
| 10CuY200 | 3.30 | 1.39 | 1.31 | 3.39 | 9.39 |
| 10CuY250 | 3.38 | 1.40 | 0.98 | 3.76 | 9.52 |
| 10CuY280 | 2.78 | 0.91 | 2.68 | 3.04 | 9.41 |
Table 1 Cu species content of 10CuYT catalysts
| Catalyst | Mass fraction(%) | ||||
|---|---|---|---|---|---|
| Cu2+(Supercage) | Cu2+(Sodalite cage) | CuO | Cu+ | Cusum | |
| 10CuY170 | 2.99 | 0.84 | 2.36 | 3.30 | 9.49 |
| 10CuY200 | 3.30 | 1.39 | 1.31 | 3.39 | 9.39 |
| 10CuY250 | 3.38 | 1.40 | 0.98 | 3.76 | 9.52 |
| 10CuY280 | 2.78 | 0.91 | 2.68 | 3.04 | 9.41 |
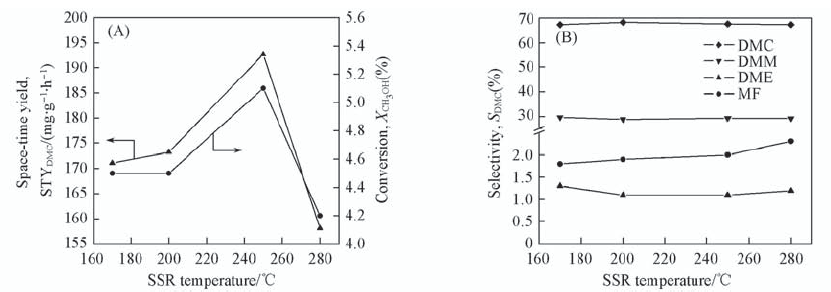
Fig.3 STYDMC and conversion(A) and selectivities of DMC, DME, DMM and MF(B) vs. SSR temperature Feed composition(volume fraction): 31.8%CH3OH, 62.5%CO, 5.7%O2; GHSV 3250 h-1.
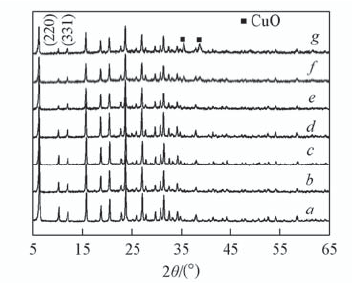
Fig.4 XRD patterns of NH4Y and catalysts with different copper loadings a. NH4Y; b. 6CuY250; c. 8CuY250; d. 10CuY250; e. 12CuY250; f. 15CuY250; g. 20CuY250.
| Catalyst | SBET/(m2·g-1) | Smicro/(m2·g-1) | Smeso/(m2·g-1) | Vmicro/(cm3·g-1) | Vmeso/(cm3·g-1) |
|---|---|---|---|---|---|
| NH4Y | 791.9 | 746.4 | 45.5 | 0.36 | 0.10 |
| 6CuY250 | 737.5 | 690.9 | 46.7 | 0.34 | 0.13 |
| 8CuY250 | 718.0 | 668.4 | 49.6 | 0.33 | 0.18 |
| 10CuY250 | 705.0 | 656.3 | 48.9 | 0.32 | 0.12 |
| 12CuY250 | 560.3 | 514.2 | 46.1 | 0.26 | 0.12 |
| 15CuY250 | 549.6 | 507.9 | 41.7 | 0.25 | 0.12 |
| 20CuY250 | 530.1 | 481.9 | 48.2 | 0.24 | 0.12 |
Table 2 Specific surface area and pore volume of catalysts with different Cu loadings*
| Catalyst | SBET/(m2·g-1) | Smicro/(m2·g-1) | Smeso/(m2·g-1) | Vmicro/(cm3·g-1) | Vmeso/(cm3·g-1) |
|---|---|---|---|---|---|
| NH4Y | 791.9 | 746.4 | 45.5 | 0.36 | 0.10 |
| 6CuY250 | 737.5 | 690.9 | 46.7 | 0.34 | 0.13 |
| 8CuY250 | 718.0 | 668.4 | 49.6 | 0.33 | 0.18 |
| 10CuY250 | 705.0 | 656.3 | 48.9 | 0.32 | 0.12 |
| 12CuY250 | 560.3 | 514.2 | 46.1 | 0.26 | 0.12 |
| 15CuY250 | 549.6 | 507.9 | 41.7 | 0.25 | 0.12 |
| 20CuY250 | 530.1 | 481.9 | 48.2 | 0.24 | 0.12 |
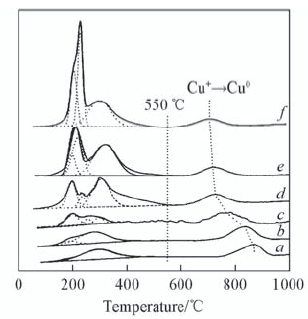
Fig.7 Hydrogen TPR profiles for the catalysts with different copper loadings a. 6CuY250; b. 8CuY250; c. 10CuY250; d. 12CuY250;e. 15CuY250; f. 20CuY250.
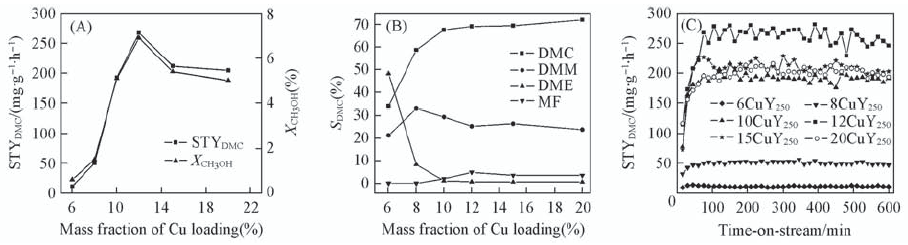
Fig.9 Influence on catalysis of copper loading STYDMC and XCH3OH vs. copper loading(A), selectivities of DMC, DME, DMM and MF vs. copper loading(B) and time-on-stream performance of xCuY250 catalysts(C) Feed composition(volume fraction): 31.8% CH3OH, 62.5% CO, 5.7% O2; GHSV 3250 h-1.
| Catalyst | Preparation method | Mass fraction of Cu loading(%) | STYDMC /(mg·g-1·h-1) | SDMC(%) | |
|---|---|---|---|---|---|
| 6CuY250 | SSR | 6.1 | 11.3a | 33.9 | 0.6 |
| 8CuY250 | SSR | 8.0 | 50.5a | 58.7 | 1.5 |
| 10CuY250 | SSR | 9.5 | 192.7a | 67.6 | 5.1 |
| 12CuY250 | SSR | 11.3 | 267.3a | 69.2 | 6.9 |
| 15CuY250 | SSR | 13.9 | 211.7a | 69.6 | 5.4 |
| 20CuY250 | SSR | 16.1 | 204.5a | 72.1 | 5.0 |
| CuHY-3[ | IEIM | 11.9 | 200.5b | 51.7 | 13.0 |
| CuCl2/HY[ | SSIE | 12.2 | 97.3b | 74.6 | 4.4 |
| Cu2(OH)3Cl/AC[ | DP | 18.7 | 139.1b | 67.3 | 6.9 |
| 12Cu- | DP | 12.0 | 152.1c | 56.3 | 8.4 |
Table 3 Catalytic activities of CuY catalysts in the oxidative carbonylation of methanol
| Catalyst | Preparation method | Mass fraction of Cu loading(%) | STYDMC /(mg·g-1·h-1) | SDMC(%) | |
|---|---|---|---|---|---|
| 6CuY250 | SSR | 6.1 | 11.3a | 33.9 | 0.6 |
| 8CuY250 | SSR | 8.0 | 50.5a | 58.7 | 1.5 |
| 10CuY250 | SSR | 9.5 | 192.7a | 67.6 | 5.1 |
| 12CuY250 | SSR | 11.3 | 267.3a | 69.2 | 6.9 |
| 15CuY250 | SSR | 13.9 | 211.7a | 69.6 | 5.4 |
| 20CuY250 | SSR | 16.1 | 204.5a | 72.1 | 5.0 |
| CuHY-3[ | IEIM | 11.9 | 200.5b | 51.7 | 13.0 |
| CuCl2/HY[ | SSIE | 12.2 | 97.3b | 74.6 | 4.4 |
| Cu2(OH)3Cl/AC[ | DP | 18.7 | 139.1b | 67.3 | 6.9 |
| 12Cu- | DP | 12.0 | 152.1c | 56.3 | 8.4 |
| [1] | Xu J., Long K. Z., Wang Y., Xue B. Li Y. X., Appl. Catal. A,2015, 496, 1—8 |
| [2] | Ren J., Wang D. L., Pei Y. L., Qin Z. F., Lin J. Y., Li Z., Chem. J. Chinese Universities,2013, 34(11), 2594—2600 |
| (任军, 王冬蕾, 裴永丽, 秦志峰, 林建英, 李忠. 高等学校化学学报,2013, 34(11), 2594—2600) | |
| [3] | Keller N., Rebmann G. Keller V., J. Mol. Catal. A,2010, 317(1/2), 1—18 |
| [4] | Wang P., Liu S., Zhou F., Yang B., Alshammari A. S., Lu L. Deng Y., Fuel Process. Technol., 2014, 126, 359—365 |
| [5] | Delledonne D., Rivetti F. Romano U., Appl. Catal. A,2001, 221(1/2), 241—251 |
| [6] | Zhu D., Mei F., Chen L., Li T., Mo W., Li G., Energy Fuels,2009, 23(5), 2359—2363 |
| [7] | Li Z., Wen C. M., Zheng H. Y., Xie K. C., Chem. J. Chinese Universities,2010, 31(1), 145—152 |
| (李忠, 文春梅, 郑华艳, 谢克昌. 高等学校化学学报,2010, 31(1), 145—152) | |
| [8] | Saada R., Kellici S., Heil T., Morgan D. Saha B., Appl. Catal. B,2015, 168/169, 353—362 |
| [9] | Du G. F., Guo H., Wang Y., Li W. J., Shi W. J., Dai B., J. Saudi Chemical Society,2015, 19(1), 112—115 |
| [10] | Nam J. K., Choi M. J., Cho D. H., Suh J. K., Kim S. B., J. Mol. Catal. A,2013, 370, 7—13 |
| [11] | King S. T., J. Catal., 1996, 161(2), 530—538 |
| [12] | King S. T., Catal. Today, 1997, 33(1—3), 173—182 |
| [13] | Li Z., Fu T.J., Zheng H. Y.,Chinese J. Inorg. Chem., 2011, (8), 1483—1490 |
| (李忠, 付廷俊, 郑华艳. 无机化学学报, 2011, (8), 1483—1490) | |
| [14] | Li Z., Fu T. J., Wang R. Y., Niu Y. Y., Zheng H. Y., Chem. J. Chinese Universities,2011, 32(6), 1366—1372 |
| (李忠, 付廷俊, 王瑞玉, 牛燕燕, 郑华艳. 高等学校化学学报,2011, 32(6), 1366—1372) | |
| [15] | Richter M., Fait M. J. G., Eckelt R., Schreier E., Schneider M., Pohl M. M., Fricke R., Appl. Catal. B,2007, 73(3/4), 269—281 |
| [16] | Richter M., Fait M. J. G., Eckelt R., Schneider M., Radnik J., Heidemann D., Fricke R., J. Catal., 2007, 245(1), 11—24 |
| [17] | Drake I. J., Zhang Y., Briggs D., Lim B., Chau T., Bell A. T., J. Phys. Chem. B,2006, 110(24), 11654—11664 |
| [18] | Zhang Y. Bell A. T., J. Catal., 2008, 255 (2), 153—161 |
| [19] | Zheng H., Qi J., Zhang R., Li Z., Wang B., Ma X., Fuel Process. Technol., 2014, 128, 310—318 |
| [20] | Zhang Y. Q., Zheng H. Y., Zhang R. G., Li Z., Wang B. J., Zhao Q. Y., Chem. J. Chinese Universities,2015, 36(10), 1945—1953 |
| (张艳表, 郑华艳, 章日光, 李忠, 王宝俊, 赵秋男. 高等学校化学学报,2015, 36(10), 1945—1953) | |
| [21] | Engeldinger J., Domke C., Richter M., Bentrup U., Appl. Catal. A,2010, 382(2), 303—311 |
| [22] | Engeldinger J., Richter M. Bentrup U., Phys. Chem. Chem. Phys., 2012, 14(7), 2183—2191 |
| [23] | Ribeiro da Silva M. A. V., Monte M. J. S. Huinink J., J. Chem. Thermodynamics,1995, 27(2), 175—190 |
| [24] | Goel P., Duragasi G. Singh J. P.,J. Mater. Sci., 2013, 48(14), 4876—4882 |
| [25] | Nasibulin A. G., Richard O., Kauppinen E. I., Brown D. P., Jokiniemi J. K. Altman I. S., Aerosol Sci. Technol., 2002, 36(8), 899—911 |
| [26] | Sun S., Wu Y. Y., Luo S. Z., Chu W., Ni H. Z., Chem. J. Chinese Universities,2011, 32(8), 1794—1798 |
| (孙思, 吴永永, 罗仕忠, 储伟, 倪宏志. 高等学校化学学报,2011, 32(8), 1794—1798) | |
| [27] | Fu T.J., Zheng H. Y., Niu Y.Y., Wang R. Y., Li Z.,Acta Chimica Sinica, 2011, (15), 1765—1772 |
| (付廷俊, 郑华艳, 牛燕燕, 王瑞玉, 李忠. 化学学报, 2011, (15), 1765—1772) | |
| [28] | Bulánek R., Wichterlová B., Sobalík Z., Tichy J., Appl. Catal. B,2001. 31(1), 13—25 |
| [29] | Sultana A., Nanba T., Sasaki M., Haneda M., Suzuki K., Hamada H., Catal. Today,2011, 164(1), 495—499 |
| [30] | Kieger S., Delahay G., Coq B., Neveu B., J. Catal., 1999, 183(2), 267—280 |
| [31] | Torre-Abreu C., Henriques C., Ribeiro F. R., Delahay G., Ribeiro M. F., Catal. Today,1999, 54(4), 407—418 |
| [32] | Song H., Wan X., Dai M., Zhang J., Li F., Song H., Fuel Process. Technol., 2013, 116, 52—62 |
| [33] | Antunes A. P., Ribeiro M. F., Silva J. M., Ribeiro F. R., Magnoux P., Guisnet M., Appl. Catal. B,2001, 33(2), 149—164 |
| [34] | Wang R.Y., Li Z.,J. Fuel Chem. Technol., 2013, (11), 1361—1366 |
| (王瑞玉, 李忠. 燃料化学学报, 2013, (11), 1361—1366) | |
| [35] | Alonso F., Melkonian T., Moglie Y., Yus M., Eur. J. Org. Chem., 2011, 2011(13), 2524—2530 |
| [36] | Li X., Zhang X., Lei L., Sep. Purif. Technol., 2009, 64(3), 326—331 |
| [37] | Zahmakıran M., Durap F., Özkar S., Int. J. Hydrogen Energy,2010, 35(1), 187—197 |
| [38] | Fei J., Hou Z., Zhu B., Lou H. Zheng X., Appl. Catal. A,2006, 304, 49—54 |
| [39] | Berthomieu D., Jardillier N., Delahay G., Coq B. Goursot A., Catal. Today,2005, 110(3/4), 294—302 |
| [40] | Wang J.Z., Study on Controlling Cu Catalytic Active Center and Catalytic Performance of CuY Catalyst, Taiyuan University of Technology, 2013 |
| (王佳臻. CuY催化剂中Cu活性中心调控及催化性能的研究, 太原: 太原理工大学, 2013 ) | |
| [41] | Li Z., Wang R. Y., Zheng H. Y., Xie K. C., Fuel, 2010, 89(7), 1339—1343 |
| [42] | Wang R.Y., Li Z., Zheng H. Y., Xie K. C.,Chinese. J. Catal., 2009, (10), 1068—1072 |
| (王瑞玉, 李忠, 郑华艳, 谢克昌.催化学报, 2009, (10), 1068—1072) |
| [1] | WANG Man, WANG Xin, ZHOU Jing, GAO Guohua. Efficient Synthesis of Dimethyl Carbonate via Transesterification of Methanol and Ethylene Carbonate Catalyzed by Poly(ionic liquid)s [J]. Chem. J. Chinese Universities, 2021, 42(12): 3701. |
| [2] | ZHANG Guoqiang, SUN Yuchen, SHI Yabo, ZHENG Huayan, LI Zhong, SHANGGUAN Ju, LIU Shoujun, SHI Pengzheng. Surface Properties of Ce1-xMnxO2 Catalyst on the Catalytic Activities for Direct Synthesis of DMC from CO2 and Methanol [J]. Chem. J. Chinese Universities, 2020, 41(9): 2061. |
| [3] | YIN Jiao, ZHANG Guoqiang, YAN Lifei, JIA Dongsen, ZHENG Huayan, LI Zhong. Influence of Structure Evolution of CuY Catalyst During the Reaction Process on Its Catalytic Performance for Oxidative Carbonylation of Methanol† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1510. |
| [4] | ZHANG Yanqing, ZHENG Huayan, ZHANG Riguang, LI Zhong, WANG Baojun, ZHAO Qiuyong. Density Functional Theory Investigation on the Effect of Alkali Metal Cations on the Catalytic Performance for Cu+Y Zeolites in Oxidative Carbonylation of Methanol† [J]. Chem. J. Chinese Universities, 2015, 36(10): 1945. |
| [5] | REN Jun, WANG Dong-Lei, PEI Yong-Li, QIN Zhi-Feng, LIN Jian-Ying, LI Zhong. Effects of Lithium Content on the Structural Properties and Catalytic Activities of CuLi/AC Catalysts in the Oxidative Carbonylation of Methanol to Dimethyl Carbonate [J]. Chem. J. Chinese Universities, 2013, 34(11): 2594. |
| [6] | PEI Yi-Xia, LI Hui-Quan, LIU Hai-Tao, ZHANG Yi. FTIR Spectroscopic Studies on Catalytic Mechanism of Lead Compounds in the Reaction of Methylene Dianiline and Dimethyl Carbonate [J]. Chem. J. Chinese Universities, 2012, 33(03): 598. |
| [7] | DU Zhi-Ping* , XIAO Yan-Hua, WANG Gong-Ying. Synthesis of Diphenyl Carbonate from the Transesterification Catalyzed by Dibutyltin Sulfonates [J]. Chem. J. Chinese Universities, 2009, 30(9): 1798. |
| [8] | LI Zhong*, WEN Chun-Mei, WANG Rui-Yu, ZHENG Hua-Yan, XIE Ke-Chang. Chloride-free Cu2O/AC Catalyst Prepared by Pyrolysis of Copper Acetate and Catalytic Oxycarbonylation [J]. Chem. J. Chinese Universities, 2009, 30(10): 2024. |
| [9] | YU Qin-Qin, WANG Shu, BAI Rong-Xian, MEI Fu-Ming, LI Guang-Xing . Transesterification of Dimethyl Carbonate and Phenol Catalyzed by Zn-Al Hydrotalcite [J]. Chem. J. Chinese Universities, 2005, 26(8): 1502. |
| [10] | ZHONG Shun-He, CHENG Qing-Yan, LI Han-Sheng . Preparation and Characterization of Supported Catalyst Sn2(OMe)2Cl2/SiO2 and Its Catalytic Properties for Synthesis of Dimethyl Carbonate [J]. Chem. J. Chinese Universities, 2003, 24(1): 125. |
| [11] | WANG Shao-Cheng, CAO Yong, YANG Ping, HU Jian-Guo, WU Dong, SUN Yu-Han, DENG Jing-Fa . TBAB-modified Supported Wacker-type Catalysts for Highly Selective Oxidative Carbonylation of Methanol to Dimethyl Carbonate [J]. Chem. J. Chinese Universities, 2002, 23(12): 2363. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||