

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (1): 126.doi: 10.7503/cjcu20150442
• Physical Chemistry • Previous Articles Next Articles
CHEN Gang1,*( ), MI Cangen1, LÜ Hong2,*, HAO Chuanpu2, HUANG Yu1, SONG Yukun2
), MI Cangen1, LÜ Hong2,*, HAO Chuanpu2, HUANG Yu1, SONG Yukun2
Received:2015-06-04
Online:2016-01-10
Published:2015-12-20
Contact:
CHEN Gang,LÜ Hong
E-mail:lvhong@tongji.edu.cn
Supported by:CLC Number:
TrendMD:
CHEN Gang, MI Cangen, LÜ Hong, HAO Chuanpu, HUANG Yu, SONG Yukun. Mesoporous TiO2 as the Anode Catalyst Support for Solid Polymer Electrolyte Water Electrolysis†[J]. Chem. J. Chinese Universities, 2016, 37(1): 126.
| Sample | Calcination temperature/℃ | SBET/(m2·g-1) | Vp/(cm3·g-1) | Pore diameter/nm |
|---|---|---|---|---|
| TiO2-1 | 300 | 172 | 0.040 | 5.2 |
| TiO2-2 | 350 | 126 | 0.026 | 6.8 |
| TiO2-3 | 450 | 36 | 0.004 | 13.4 |
Table 1 BET parameters of calcined TiO2 samples
| Sample | Calcination temperature/℃ | SBET/(m2·g-1) | Vp/(cm3·g-1) | Pore diameter/nm |
|---|---|---|---|---|
| TiO2-1 | 300 | 172 | 0.040 | 5.2 |
| TiO2-2 | 350 | 126 | 0.026 | 6.8 |
| TiO2-3 | 450 | 36 | 0.004 | 13.4 |
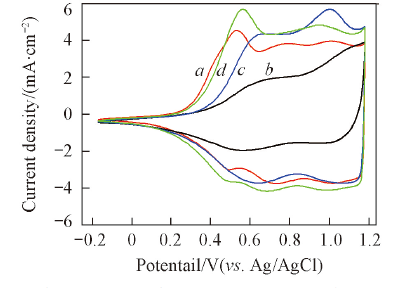
Fig.8 CV curves of catalyst samples in N2-satura-ted 0.5 mol/L H2SO4 solution at room temperature(scan rate: 50 mV/s)^a. IrO2; b. 40%IrO2/TiO2-1; c. 40%IrO2/TiO2-2; d. 40%IrO2/TiO2-3.
| Sample | RΩ/(Ω·cm2) | CPE/(mF·cm-2) | Rct/(Ω·cm2) | n |
|---|---|---|---|---|
| IrO2 | 0.40 | 143 | 0.240 | 0.926 |
| 40%IrO2/TiO2-2 | 0.74 | 102 | 0.400 | 0.814 |
| 40%IrO2/TiO2-3 | 0.57 | 117 | 0.385 | 0.883 |
Table 2 Impendence parameters obtained from fitting the experimental data to the RΩ(RctCPE) circuit measured at 0.1 A/cm2*
| Sample | RΩ/(Ω·cm2) | CPE/(mF·cm-2) | Rct/(Ω·cm2) | n |
|---|---|---|---|---|
| IrO2 | 0.40 | 143 | 0.240 | 0.926 |
| 40%IrO2/TiO2-2 | 0.74 | 102 | 0.400 | 0.814 |
| 40%IrO2/TiO2-3 | 0.57 | 117 | 0.385 | 0.883 |
| [1] | Troncoso E., Newborough M., Int. J. Hydrogen Energy, 2011, 36(1), 120—134 |
| [2] | Ipsakis D., Voutetakis S., Seferlis P., Stergiopoulos F., Elmasides C., Int. J. Hydrogen Energy, 2009, 34(16), 7081—7095 |
| [3] | Barbir F., Sol. Energy, 2005, 78(5), 661—669 |
| [4] | Lu P. W. T., Srinivasan S., J. Appl. Electro. Chem., 1979, 9(3), 269—283 |
| [5] | Holladay J. D., Hu J., King D. L., Wang Y., Catal. Today, 2009, 139(4), 244—260 |
| [6] | Andolfatto F., Durand R., Michas A., Millet P., Stevens P., Int. J. Hydrogen Energy, 1994, 19(5), 421—427 |
| [7] | Grigoriev S. A., Porembsky V. I., Fateev V. N., Int. J. Hydrogen Energy, 2006, 31(2), 171—175 |
| [8] | Siracusano S., Baglio V., Di Blasi A., Briguglio N., Stassi A., Ornelas R., Arico A. S., Int. J. Hydrogen Energy, 2010, 35(11), 5558—5568 |
| [9] | Da Silva L. M., Boodts J. F. C., DeFaria L. A., Electrochimica Acta, 2000, 45(17), 2719—2727 |
| [10] | Kötz R., Stucki S., Electrochimca Acta, 1986, 31(10), 1311—1316 |
| [11] | Mamaca N., Mayousse E., Arrii-Clacens S., Napporn T. W., Servat K., Guillet N., Kokoh K. B., Appl. Catal. B-Environ., 2012, 111, 376—380 |
| [12] | Song S., Zhang H., Ma X., Shao Z., Baker R. T., Yi B., Int. J. Hydrogen Energy, 2008, 33(19), 4955—4961 |
| [13] | Siracusano S., Baglio V., Stassi A., Ornelas R., Antonucci V., Aricò A. S., Int. J. Hydrogen Energy, 2011, 36(13), 7822—7831 |
| [14] | Huang S. Y., Ganesan P., Park S., Popov B. N., J. Am. Chem. Soc., 2009, 131(39), 13898—13899 |
| [15] | Mazúr P., Polonsky J., Paidar M., Bouzek K., Int. J. Hydrogen Energy, 2012, 37(17), 12081—12088 |
| [16] | Zhang R., Elzatahry A. A., Al-Deyab S. S., Zhao D., Nano Today, 2012, 7(4), 344—366 |
| [17] | Gao W., Wu F. Q., Luo Z., Fu J. X., Wang D. J., Xu B. K., Chem. J. Chinese Universities, 2001, 22(4), 660—662 |
| (高伟, 吴凤清, 罗臻, 富菊霞, 王德军, 徐宝琨. 高等学校化学学报, 2001, 22(4), 660—662) | |
| [18] | Wang J. Q., Xin B. F., Yu H. T., Ren Z. Y., Qu P. F., Fu H. G., Chem. J. Chinese Universities, 2003, 24(6), 1093—1096 |
| (王建强, 辛柏福, 于海涛, 任志宇, 曲鹏飞, 付宏刚. 高等学校化学学报,2003, 24(6), 1093—1096) | |
| [19] | Grosso D., Cagnol F., Soler-Illia G. D. A., Crepaldi E. L., Amenitsch H., Brunet-Bruneau A., Sanchez C., Adv. Funct. Mater., 2004, 14(4), 309—322 |
| [20] | Soler-Illia G. J. A. A., Louis A., Sanchez C., Chem. Mater., 2002, 14(2), 750—759 |
| [21] | Adams R., Voorhees V., Shriner R. L.,Org. Synth., 1941, 92 |
| [22] | Rasten E., Hagen G., Tunold R., Electrochimica Acta, 2003, 48(25), 3945—3952 |
| [23] | Cheng J., Zhang H., Ma H., Zhong H., Zou Y., Int. J. Hydrogen Energy, 2009, 34(16), 6609—6613 |
| [24] | Birks L. S., Friedman H., J. Appl. Phys., 1946, 17(8), 687—692 |
| [25] | Kong F. D., Zhang S., Yin G. P., Wang Z. B., Du C. Y., Chen G. Y., Zhang N., Int. J. Hydrogen Energy, 2012, 37(1), 59—67 |
| [26] | Li G., Yu H., Wang X., Sun S., Li Y., Shao Z., Yi B., Phys. Chem. Chem. Phys., 2013, 15(8), 2858—2866 |
| [27] | Marshall A., Børresen B., Hagen G., Sunde S., Tsypkin M., Tunold R., Russ. J. Electrochem., 2006, 42(10), 1134—1140 |
| [28] | Puthiyapura V. K., Mamlouk M., Pasupathi S., Pollet B. G., J. Power Sources, 2014, 269, 451—460 |
| [29] | Fachinotti E., Guerrini E., Tavares A. C., Trasatti S., J. Electroanal. Chem., 2007, 600(1), 103—112 |
| [1] | LIN Gaoxin, WANG Jiacheng. Progress and Perspective on Molybdenum Disulfide with Single-atom Doping Toward Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220321. |
| [2] | TANG Quanjun, LIU Yingxin, MENG Rongwei, ZHANG Ruotian, LING Guowei, ZHANG Chen. Application of Single-atom Catalysis in Marine Energy [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220324. |
| [3] | FAN Jianling, TANG Hao, QIN Fengjuan, XU Wenjing, GU Hongfei, PEI Jiajing, CEHN Wenxing. Nitrogen Doped Ultra-thin Carbon Nanosheet Composited Platinum-ruthenium Single Atom Alloy Catalyst for Promoting Electrochemical Hydrogen Evolution Process [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220366. |
| [4] | WEI Chunhong, JIANG Qian, WANG Panpan, JIANG Chengfa, LIU Yuefeng. Atomic Scale Investigation of Pt Atoms/clusters Promoted Co-catalyzed Fischer-Tropsch Synthesis [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220074. |
| [5] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [6] | ZHANG Xinxin, XU Di, WANG Yanqiu, HONG Xinlin, LIU Guoliang, YANG Hengquan. Effect of Mn Promoter on CuFe-based Catalysts for CO2 Hydrogenation to Higher Alcohols [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220187. |
| [7] | DING Yang, WANG Wanhui, BAO Ming. Recent Progress in Porous Framework-immobilized Molecular Catalysts for CO2 Hydrogenation to Formic Acid [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220309. |
| [8] | ZHOU Leilei, CHENG Haiyang, ZHAO Fengyu. Research Progress of CO2 Hydrogenation over Pd-based Heterogeneous Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220279. |
| [9] | HUANG Xiaoshun, MA Haiying, LIU Shujuan, WANG Bin, WANG Hongli, QIAN Bo, CUI Xinjiang, SHI Feng. Recent Advances on Indirect Conversion of Carbon Dioxide to Chemicals [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220222. |
| [10] | MIN Jing, WANG Liyan. 1H NMR Study on the Conformation of Aromatic Amides Limited by Three-center Hydrogen Bonds [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220084. |
| [11] | SONG Youwei, AN Jiangwei, WANG Zheng, WANG Xuhui, QUAN Yanhong, REN Jun, ZHAO Jinxian. Effects of Ag,Zn,Pd-doping on Catalytic Performance of Copper Catalyst for Selective Hydrogenation of Dimethyl Oxalate [J]. Chem. J. Chinese Universities, 2022, 43(6): 20210842. |
| [12] | YANG Lijun, YU Yang, ZHANG Lei. Construction of Dual-functional 2D/3D Hydrid Co2P-CeO x Heterostructure Integrated Electrode for Electrocatalytic Urea Oxidation Assisted Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220082. |
| [13] | WANG Guangqi, BI Yiyang, WANG Jiabo, SHI Hongfei, LIU Qun, ZHANG Yu. Heterostructure Construction of Noble-metal-free Ternary Composite Ni(PO3)2-Ni2P/CdS NPs and Its Visible Light Efficient Catalytic Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220050. |
| [14] | CHEN Changli, MI Wanliang, LI Yujing. Research Progress of Single Atom Catalysts in Electrochemical Hydrogen Cycling [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220065. |
| [15] | HU Huimin, CUI Jing, LIU Dandan, SONG Jiaxin, ZHANG Ning, FAN Xiaoqiang, ZHAO Zhen, KONG Lian, XIAO Xia, XIE Zean. Influence of Different Transition Metal Decoration on the Propane Dehydrogenation Performance over Pt/M-DMSN Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210815. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||