

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (10): 1954.doi: 10.7503/cjcu20150326
• Physical Chemistry • Previous Articles Next Articles
Received:2015-04-22
Online:2015-10-10
Published:2015-09-14
Contact:
FANG Decai
E-mail:dcfang@bnu.edu.cn
Supported by:CLC Number:
TrendMD:
LI Yue, FANG Decai. Density Functional Theory Studies on the t-Butoxyl Radical Mediated Hydrogen Atom Transfer Reactions†[J]. Chem. J. Chinese Universities, 2015, 36(10): 1954.
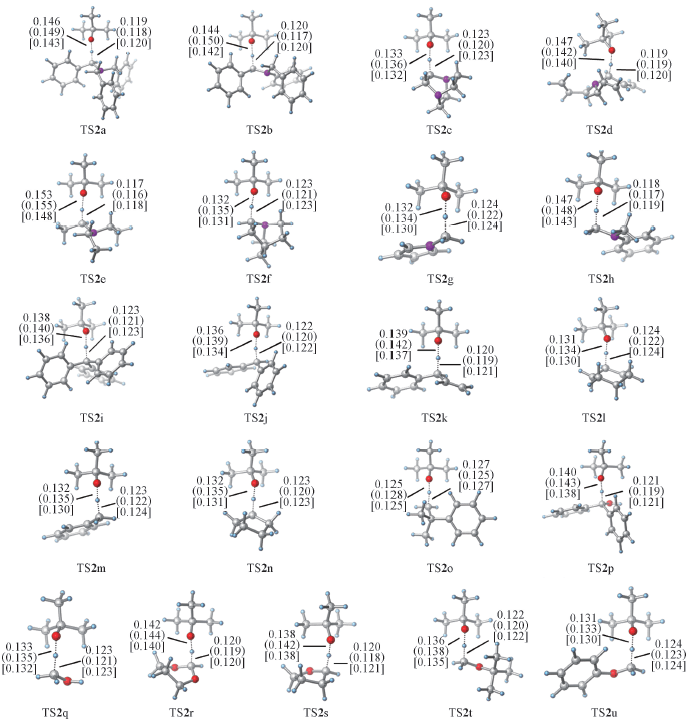
Fig.1 Main geometric parameters(nm) of 21 transition states in benzene solution, obtained with CAM-B3LYP, M062x and wB97x from top to bottom, respectively2a—2h: Amines; 2i—2o: hydrocarbons; 2p—2q: alcohols; 2r—2u: ethers.
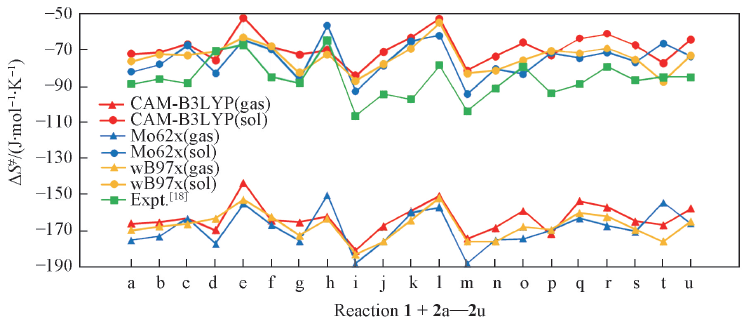
Fig.2 Calculated activation entropies(ΔS≠) for 21 hydrogen abstractions(1+2a—2u) obtained with gas-phase translational entropies ΔS≠(gas) for those methods and with solution translational entropies ΔS≠(sol) for those methods with PCM(in THF), along with experimental measurements[18]
| Species | Reaction | CAM-B3LYPa | CAM-B3LYPb | M062xa | wB97xa | Expt.c | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΔG≠(g)d/ (kJ·mol-1) | ΔG≠(l)e/ (kJ·mol-1) | ΔG≠(g) / (kJ·mol-1) | ΔG≠(l)/ (kJ·mol-1) | ΔG≠(g)/ (kJ·mol-1) | ΔG≠(l)/ (kJ·mol-1) | ΔG≠(g)/ (kJ·mol-1) | ΔG≠(l)/ (kJ·mol-1) | |||
| Amines | 1+2a | 51.0 | 23.0 | 57.3 | 28.4 | 43.1 | 15.1 | 48.5 | 20.5 | 29.7 |
| 1+2b | 54.4 | 26.3 | 61.1 | 31.8 | 48.1 | 19.7 | 53.9 | 25.5 | 31.4 | |
| 1+2c | 64.0 | 35.1 | 63.6 | 35.1 | 56.5 | 28.0 | 65.7 | 37.2 | 32.6 | |
| 1+2d | 55.6 | 27.6 | 66.9 | 38.5 | 53.1 | 24.7 | 54.4 | 26.8 | 27.6 | |
| 1+2e | 45.6 | 18.4 | 51.4 | 23.8 | 43.5 | 16.3 | 47.3 | 20.5 | 27.6 | |
| 1+2f | 65.7 | 36.8 | 65.6 | 36.4 | 57.7 | 28.9 | 64.4 | 36.4 | 33.0 | |
| 1+2g | 70.7 | 43.1 | 68.6 | 40.6 | 63.6 | 36.8 | 72.8 | 45.6 | 33.9 | |
| 1+2h | 51.4 | 24.3 | 53.1 | 24.7 | 48.5 | 20.1 | 53.9 | 27.2 | 27.2 | |
| Hydrocarbons | 1+2i | 67.7 | 38.9 | 76.1 | 47.7 | 54.8 | 26.3 | 64.4 | 35.5 | 36.8 |
| 1+2j | 66.5 | 37.6 | 69.0 | 39.7 | 59.0 | 30.1 | 68.2 | 38.9 | 35.5 | |
| 1+2k | 63.6 | 34.7 | 63.1 | 33.9 | 57.7 | 29.3 | 65.2 | 36.8 | 36.0 | |
| 1+2l | 70.3 | 40.6 | 68.6 | 39.3 | 63.6 | 35.1 | 69.4 | 40.6 | 39.3 | |
| 1+2m | 77.8 | 49.8 | 74.9 | 46.4 | 74.0 | 46.0 | 78.2 | 50.2 | 43.1 | |
| 1+2n | 72.8 | 44.3 | 71.5 | 42.2 | 68.2 | 39.7 | 74.9 | 46.4 | 39.3 | |
| 1+2o | 83.2 | 55.6 | 82.8 | 55.2 | 75.7 | 48.5 | 85.3 | 57.7 | 46.8 | |
| Alcohols | 1+2p | 50.2 | 20.9 | 58.1 | 27.6 | 42.2 | 13.0 | 48.5 | 18.8 | 33.9 |
| 1+2q | 61.1 | 34.3 | 61.5 | 34.3 | 61.5 | 35.1 | 64.4 | 37.6 | 46.0 | |
| Ethers | 1+2r | 45.6 | 17.1 | 48.9 | 20.1 | 45.2 | 16.3 | 47.3 | 19.2 | 33.5 |
| 1+2s | 53.1 | 23.8 | 53.9 | 25.1 | 49.3 | 21.3 | 53.5 | 25.5 | 33.9 | |
| 1+2t | 63.6 | 36.8 | 62.7 | 35.5 | 56.4 | 30.1 | 65.7 | 39.3 | 44.3 | |
| 1+2u | 67.3 | 39.3 | 68.6 | 40.1 | 63.1 | 35.1 | 69.4 | 42.2 | 44.7 | |
Table 1 Calculated free-energy barriers obtained by different methods in benzene solution(298.15 K)
| Species | Reaction | CAM-B3LYPa | CAM-B3LYPb | M062xa | wB97xa | Expt.c | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΔG≠(g)d/ (kJ·mol-1) | ΔG≠(l)e/ (kJ·mol-1) | ΔG≠(g) / (kJ·mol-1) | ΔG≠(l)/ (kJ·mol-1) | ΔG≠(g)/ (kJ·mol-1) | ΔG≠(l)/ (kJ·mol-1) | ΔG≠(g)/ (kJ·mol-1) | ΔG≠(l)/ (kJ·mol-1) | |||
| Amines | 1+2a | 51.0 | 23.0 | 57.3 | 28.4 | 43.1 | 15.1 | 48.5 | 20.5 | 29.7 |
| 1+2b | 54.4 | 26.3 | 61.1 | 31.8 | 48.1 | 19.7 | 53.9 | 25.5 | 31.4 | |
| 1+2c | 64.0 | 35.1 | 63.6 | 35.1 | 56.5 | 28.0 | 65.7 | 37.2 | 32.6 | |
| 1+2d | 55.6 | 27.6 | 66.9 | 38.5 | 53.1 | 24.7 | 54.4 | 26.8 | 27.6 | |
| 1+2e | 45.6 | 18.4 | 51.4 | 23.8 | 43.5 | 16.3 | 47.3 | 20.5 | 27.6 | |
| 1+2f | 65.7 | 36.8 | 65.6 | 36.4 | 57.7 | 28.9 | 64.4 | 36.4 | 33.0 | |
| 1+2g | 70.7 | 43.1 | 68.6 | 40.6 | 63.6 | 36.8 | 72.8 | 45.6 | 33.9 | |
| 1+2h | 51.4 | 24.3 | 53.1 | 24.7 | 48.5 | 20.1 | 53.9 | 27.2 | 27.2 | |
| Hydrocarbons | 1+2i | 67.7 | 38.9 | 76.1 | 47.7 | 54.8 | 26.3 | 64.4 | 35.5 | 36.8 |
| 1+2j | 66.5 | 37.6 | 69.0 | 39.7 | 59.0 | 30.1 | 68.2 | 38.9 | 35.5 | |
| 1+2k | 63.6 | 34.7 | 63.1 | 33.9 | 57.7 | 29.3 | 65.2 | 36.8 | 36.0 | |
| 1+2l | 70.3 | 40.6 | 68.6 | 39.3 | 63.6 | 35.1 | 69.4 | 40.6 | 39.3 | |
| 1+2m | 77.8 | 49.8 | 74.9 | 46.4 | 74.0 | 46.0 | 78.2 | 50.2 | 43.1 | |
| 1+2n | 72.8 | 44.3 | 71.5 | 42.2 | 68.2 | 39.7 | 74.9 | 46.4 | 39.3 | |
| 1+2o | 83.2 | 55.6 | 82.8 | 55.2 | 75.7 | 48.5 | 85.3 | 57.7 | 46.8 | |
| Alcohols | 1+2p | 50.2 | 20.9 | 58.1 | 27.6 | 42.2 | 13.0 | 48.5 | 18.8 | 33.9 |
| 1+2q | 61.1 | 34.3 | 61.5 | 34.3 | 61.5 | 35.1 | 64.4 | 37.6 | 46.0 | |
| Ethers | 1+2r | 45.6 | 17.1 | 48.9 | 20.1 | 45.2 | 16.3 | 47.3 | 19.2 | 33.5 |
| 1+2s | 53.1 | 23.8 | 53.9 | 25.1 | 49.3 | 21.3 | 53.5 | 25.5 | 33.9 | |
| 1+2t | 63.6 | 36.8 | 62.7 | 35.5 | 56.4 | 30.1 | 65.7 | 39.3 | 44.3 | |
| 1+2u | 67.3 | 39.3 | 68.6 | 40.1 | 63.1 | 35.1 | 69.4 | 42.2 | 44.7 | |
| Species | Reaction | k/(L·mol-1·s-1) | |||
|---|---|---|---|---|---|
| CAM-B3LYP | M062x | wB97x | Expt.[ | ||
| Amines | 1+2a | 6.1×108 | 1.4×1010 | 1.5×109 | 4.2×107 |
| 1+2b | 1.5×108 | 2.1×109 | 2.2×108 | 1.9×107 | |
| 1+2c | 4.2×106 | 7.5×107 | 1.8×106 | 1.2×107 | |
| 1+2d | 9.3×107 | 2.7×108 | 1.2×108 | 8.9×107 | |
| 1+2e | 3.9×109 | 8.9×109 | 1.6×109 | 9.8×107 | |
| 1+2f | 2.2×106 | 5.6×107 | 2.8×106 | 1.0×107 | |
| 1+2g | 1.8×105 | 2.2×106 | 6.2×104 | 7.3×106 | |
| 1+2h | 3.8×108 | 1.8×109 | 1.1×108 | 1.0×108 | |
| Hydrocarbons | 1+2i | 9.2×105 | 1.5×108 | 3.6×106 | 2.1×106 |
| 1+2j | 1.5×106 | 3.3×107 | 1.0×106 | 3.4×106 | |
| 1+2k | 5.3×106 | 4.8×107 | 2.2×106 | 3.2×106 | |
| 1+2l | 4.6×105 | 4.3×106 | 4.8×105 | 8.1×105 | |
| 1+2m | 1.1×104 | 5.6×104 | 9.3×103 | 1.9×105 | |
| 1+2n | 1.1×105 | 6.8×105 | 4.5×104 | 8.6×105 | |
| 1+2o | 1.2×103 | 2.0×104 | 5.0×102 | 4.0×104 | |
| Alcohols | 1+2p | 1.4×109 | 3.2×1010 | 3.2×109 | 6.9×106 |
| 1+2q | 6.5×106 | 4.6×106 | 1.5×106 | 5.3×104 | |
| Ethers | 1+2r | 6.1×109 | 8.2×109 | 2.7×109 | 7.9×106 |
| 1+2s | 4.0×108 | 1.1×109 | 2.2×108 | 7.4×106 | |
| 1+2t | 2.2×106 | 3.3×107 | 8.7×105 | 1.1×105 | |
| 1+2u | 7.9×105 | 4.2×106 | 2.7×105 | 9.5×104 | |
Table 2 Comparison for reaction rate constants obtained by different DFT methods, along with the experimental rate constants at 298.15 K
| Species | Reaction | k/(L·mol-1·s-1) | |||
|---|---|---|---|---|---|
| CAM-B3LYP | M062x | wB97x | Expt.[ | ||
| Amines | 1+2a | 6.1×108 | 1.4×1010 | 1.5×109 | 4.2×107 |
| 1+2b | 1.5×108 | 2.1×109 | 2.2×108 | 1.9×107 | |
| 1+2c | 4.2×106 | 7.5×107 | 1.8×106 | 1.2×107 | |
| 1+2d | 9.3×107 | 2.7×108 | 1.2×108 | 8.9×107 | |
| 1+2e | 3.9×109 | 8.9×109 | 1.6×109 | 9.8×107 | |
| 1+2f | 2.2×106 | 5.6×107 | 2.8×106 | 1.0×107 | |
| 1+2g | 1.8×105 | 2.2×106 | 6.2×104 | 7.3×106 | |
| 1+2h | 3.8×108 | 1.8×109 | 1.1×108 | 1.0×108 | |
| Hydrocarbons | 1+2i | 9.2×105 | 1.5×108 | 3.6×106 | 2.1×106 |
| 1+2j | 1.5×106 | 3.3×107 | 1.0×106 | 3.4×106 | |
| 1+2k | 5.3×106 | 4.8×107 | 2.2×106 | 3.2×106 | |
| 1+2l | 4.6×105 | 4.3×106 | 4.8×105 | 8.1×105 | |
| 1+2m | 1.1×104 | 5.6×104 | 9.3×103 | 1.9×105 | |
| 1+2n | 1.1×105 | 6.8×105 | 4.5×104 | 8.6×105 | |
| 1+2o | 1.2×103 | 2.0×104 | 5.0×102 | 4.0×104 | |
| Alcohols | 1+2p | 1.4×109 | 3.2×1010 | 3.2×109 | 6.9×106 |
| 1+2q | 6.5×106 | 4.6×106 | 1.5×106 | 5.3×104 | |
| Ethers | 1+2r | 6.1×109 | 8.2×109 | 2.7×109 | 7.9×106 |
| 1+2s | 4.0×108 | 1.1×109 | 2.2×108 | 7.4×106 | |
| 1+2t | 2.2×106 | 3.3×107 | 8.7×105 | 1.1×105 | |
| 1+2u | 7.9×105 | 4.2×106 | 2.7×105 | 9.5×104 | |
| [1] | Walling C., Jacknow B. B., J. Am.Chem. Soc., 1960, 82, 6108—6112 |
| [2] | Walling C., McGuiness J. A., J. Am.Chem. Soc., 1969, 91(8), 2053—2058 |
| [3] | Carter W. P. L., Darnall K. R., Lioyd A. C., Chem. Phys. Lett., 1976, 42(1), 22—27 |
| [4] | Adam W., Grimm G. N., Saha-Moeller C. R., Dall A. F., Miolo G., Daniela V., Chem. Res. Toxicology., 1998, 11, 1089—1097 |
| [5] | Adam W., Marquardt S., Kemmer D., Saha-Moeller C. R., Schreier P., Org. Lett., 2002, 4, 225—228 |
| [6] | Mahler H. C., Schulz I., Adam W., Grimm G. N., Saha-Moeller C. R., Epe B., Mutation Research, 2001, 46, 289—299 |
| [7] | Jones C. M., Burkitt M. J., J. Am. Chem. Soc., 2003, 125, 6946—6954 |
| [8] | Lindsay S. J. R., Nagatomi E., Stead A., Waddington D. J., Beviere S. D., J. Chem. Soc., Perkin Trans., 2000, 2, 1193—1198 |
| [9] | Karki S. B., Treemaneekam V., KaufmanM. J., J. Pharm. Sci., 2000, 89, 1518—1524 |
| [10] | Hartung J., Schneiders N., Gottwald T., Terahedron Lett., 2007, 48, 6027—6030 |
| [11] | Paul H., Small R. D., Scaiano J. C., J. Am. Chem. Soc., 1978, 100(14), 4520—4527 |
| [12] | Encinas M. V., Scaiano J. C., J. Am. Chem. Soc., 1981, 103(21), 6393—6397 |
| [13] | Russell G.A., Reactivity, Selectivity, and Polar Effects in Hydrogen Atom Transfer Reactions, Wiley,New York, 1973, 1—13 |
| [14] | Suleman N. K., Flores J., Tanko J. M., Isin E. M., Castaqnoli N. Jr., Bioorg. Med. Chem., 2008, 16(18), 8557—8562 |
| [15] | Tsentalovich Y. P., Kulik L. V., Gritsan N. P., Yurkovskaya A. V., J.Phys. Chem. A, 1998, 102, 7975—7980 |
| [16] | Baciocchi E., Bietti M., Salamone M., Steenken S., J. Org. Chem., 2002, 67, 2266—2270 |
| [17] | Roberts B. P., Chem. Soc. Rev., 1999, 28, 25—35 |
| [18] | Finn M., Friegline R., Suleman N. K., J. Am. Chem. Soc., 2004, 126(24), 7578—7584 |
| [19] | Wong S. K., J. Am. Chem. Soc., 1979, 101, 1235—1239 |
| [20] | Salamone M., Giammarioli I., Bietti M., J. Org. Chem., 2011, 76(11), 4645—4651 |
| [21] | Poleshchuk O. K., Yureva A. G., Filimonov V. D., Frenking G., Journal of Molecular Structure, 2009, 912, 67—72 |
| [22] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Gaussian Inc., Wallingford CT, 2009 |
| [23] | Lee C., Yang W., Parr R. G., Phys. Rev. B, 1988, 37, 785—789 |
| [24] | Becke A. D., J. Chem. Phys., 1993, 98, 5648—5652 |
| [25] | Yanai T., Tew D. P., Handy N. C., Chem. Phys. Lett., 2004, 393, 51—56 |
| [26] | Ditchfield R., Hehre W. J., Pople J. A., J. Chem. Phys., 1971, 54, 724—728 |
| [27] | Francl M. M., Pietro W. J., Hehre W. J., Binkley J. S., DeFrees D. J., Pople J. A., Gordon M. S., J. Chem. Phys., 1982, 77, 3654—3665 |
| [28] | Miertus S., Scrocco E., Tomasi J., Chem. Phys., 1981, 55, 117—129 |
| [29] | Scalmani G., Frisch M. J., J. Chem. Phys., 2010, 132, 114—110 |
| [30] | Tao J. Y., Mu W. H., Chass G. A., Tang T. H., Fang D. C., Int. J. Quantum Chem., 2013, 113, 975—984 |
| [31] | Zhao Y., Truhlar D. G., J. Chem. Phys., 2006, 125, 194101-1—194101-5 |
| [32] | Chai J. D., Head-Gordon M., J. Chem. Phys., 2008, 128, 084106-1—084106-15 |
| [33] | McLean A. D., Chandler G. S., J. Chem. Phys., 1980, 72, 5639—5648 |
| [34] | Raghavachari K., Binkley J. S., Seeger R., Pople J. A., J. Chem. Phys., 1980, 72, 650—654 |
| [35] | Fang D.C., Thermo Program, Beijing Normal University,Beijing, 2013 |
| [36] | Trouton F., Philosophical Magazine, 1884, 18, 54—57 |
| [37] | Atkins P., Physical Chemistry, Oxford University Press, London, 1978 |
| [38] | Liang Y., Liu S., Xia Y., Li Y., Yu Z. X., Chem. Eur. J., 2008, 14, 4361—4373 |
| [39] | Lin S. H., Lau K. H., Volk L., Richardson W., Eyring H., Proc. Natl. Acad. Sci. USA, 1972, 69, 2778—2782 |
| [40] | Volk L., Richardson W., Lau K.H., Hall M., Lin S.H., J. Chem. Ed., 1977, 54, 95—97 |
| [1] | DENG Hongri, CAO Xiaomei, WANG Jingbo, LI Xiangyuan. Rate Rules for Hydrogen Abstraction Reactions of Polycyclic Aromatic Hydrocarbons and Unsaturated Radicals [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210563. |
| [2] | WANG Jian, ZHANG Hongxing. Theoretical Study on the Structural-photophysical Relationships of Tetra-Pt Phosphorescent Emitters [J]. Chem. J. Chinese Universities, 2021, 42(7): 2245. |
| [3] | LI Xiangyuan,YAO Xiaoxia,SHENTU Jiangtao,SUN Xiaohui,LI Juanqin,LIU Mingxia,XU Shimin. Combustion Reaction Mechanism Construction by Two-parameter Rate Constant Method † [J]. Chem. J. Chinese Universities, 2020, 41(3): 512. |
| [4] | WANG Ning,ZHU Huifang,WANG Lu,ZHANG Tiantian,GU Jiali,SHU Jie. Structural Identification and Asymmetric-exchange Dynamics Study of Esomeprazole Magnesium in Specific Solution as Probed by Using 1H NMR Spectra† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1919. |
| [5] | LI Yingli, WANG Jingbo, LI Xiangyuan. Kinetic Mechanism Study on Low Temperature for Decalin Combustion† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1212. |
| [6] | FANG Sheng, LIU Jingjing, DUAN Xuemei, TAO Fuming, LIU Jingyao. Ab initio Calculation and Kinetic Investigation of Monacid-catalyzed Decomposition of Sulfurous Acid [J]. Chem. J. Chinese Universities, 2017, 38(8): 1390. |
| [7] | MA Qian, WANG Weina, ZHAO Qiangli, LIU Fengyi, WANG Wenliang. Theoretical Studies on the Reaction Mechanism of Criegee Intermediates RCHOO(R=H, CH3) with NCO Radical† [J]. Chem. J. Chinese Universities, 2017, 38(4): 613. |
| [8] |
WANG Rui, LI Yili, FENG Xukai, SONG Liang, ZHANG Tianlei, WANG Zhuqing, JIN Lingxia, ZHANG Qiang, XU Qiong, WANG Zhiyin.
Catalytic Effect of n(H2O)(n=1,2) on the Reaction of HO2+NO |
| [9] | GAO Zhifang, WANG Weina, MA Qian, LIU Fengyi, WANG Wenliang. Theoretical Studies on the Reaction Mechanism of Criegee Intermediates CH3CHOO with OH Radicals† [J]. Chem. J. Chinese Universities, 2016, 37(3): 513. |
| [10] | ZHU Peng, DUAN Xuemei, LIU Jingyao. Mechanism and Kinetics of the Hydrogen-abstraction Reaction of CF2ClC(O)OCH2CH3 with OH Radicals† [J]. Chem. J. Chinese Universities, 2016, 37(1): 79. |
| [11] | WANG Kuan, CHEN Jiangang, WANG Bozhou, LU Jian, WANG Wenliang, LIU Fengyi, ZHOU Cheng, LIAN Peng, LIU Zhongwen, LIU Zhaotie. Mechanisms and Kinetics of the Synthesis of FOX-12† [J]. Chem. J. Chinese Universities, 2015, 36(3): 531. |
| [12] | HU Xixi, YANG Junying, XIE Daiqian. State-to-state Quantum Dynamics of Reaction N+NH→N2+H [J]. Chem. J. Chinese Universities, 2015, 36(11): 2198. |
| [13] | MA Peng, SONG Jinou, SONG Chonglin, LÜ Gang, CHEN Chaoxu, YANG Chuanwang. Effect of H-atom Abstraction Reactions Among C2H3, C2H5OH and CH3HCO on the Combustion of Ethanol-Hydrocarbon Fuels† [J]. Chem. J. Chinese Universities, 2015, 36(1): 149. |
| [14] | GUO Sha, WANG Weina, JIN Lingxia, WANG Shuai, WANG Wenliang. Mechanistic Studies on CH3CH2O+HCHO Reaction and Rate Constants of Major Channel† [J]. Chem. J. Chinese Universities, 2014, 35(6): 1300. |
| [15] | JIN Tong-Yin, WANG Qin, LIU Jing-Yao. Mechanism and Kinetics for Reaction of CF3CH2CF2CH3(HFC-365mfc) with Cl Atom [J]. Chem. J. Chinese Universities, 2013, 34(3): 641. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
