

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (5): 928.doi: 10.7503/cjcu20131290
• Articles: Inorganic Chemistry • Previous Articles Next Articles
LIU Chao1,*( ), ZHUO Xin1, ZHANG Hu1, LIU Xinhua2
), ZHUO Xin1, ZHANG Hu1, LIU Xinhua2
Received:2013-12-30
Online:2014-05-10
Published:2014-03-26
Contact:
LIU Chao
E-mail:ahliuchao333@163.com
Supported by:CLC Number:
TrendMD:
LIU Chao, ZHUO Xin, ZHANG Hu, LIU Xinhua. Synthesis, Crystal Structure and Catalytic Performance of Zinc Complex Containing 2-(Aminomethyl)pyridine Ligand†[J]. Chem. J. Chinese Universities, 2014, 35(5): 928.
| Empirical formula | C36H50N8O12Zn3 | Z | 2 |
|---|---|---|---|
| Formula weight | 982.95 | Dc/(g·cm-3) | 1.405 |
| Crystal system | Triclinic | μ/mm-1 | 1.612 |
| Space group | P | F(000) | 1000 |
| Temperature/K | 296(2) | Crystal size | 0.35 mm×0.32 mm×0.27 mm |
| a/nm | 1.07360(19) | θ range for data collection/(°) | 1.08—26.00 |
| b/nm | 1.2491(2) | Reflections collected/unique, Rint | 17256/8719, 0.0809 |
| c/nm | 1.8976(3) | Data/restraints/parameters | 8719/0/532 |
| α/(°) | 83.809(2) | Goodness-of-fit on F2 | 1.002 |
| β/(°) | 89.595(2) | Final R indices [I>2σ(I)] | R1=0.0694, wR2=0.1758 |
| γ/(°) | 65.708(2) | R indices(all data) | R1=0.1965, wR2=0.2323 |
| V/nm3 | 2.3039(7) |
Table 1 Crystallographic data for the complex
| Empirical formula | C36H50N8O12Zn3 | Z | 2 |
|---|---|---|---|
| Formula weight | 982.95 | Dc/(g·cm-3) | 1.405 |
| Crystal system | Triclinic | μ/mm-1 | 1.612 |
| Space group | P | F(000) | 1000 |
| Temperature/K | 296(2) | Crystal size | 0.35 mm×0.32 mm×0.27 mm |
| a/nm | 1.07360(19) | θ range for data collection/(°) | 1.08—26.00 |
| b/nm | 1.2491(2) | Reflections collected/unique, Rint | 17256/8719, 0.0809 |
| c/nm | 1.8976(3) | Data/restraints/parameters | 8719/0/532 |
| α/(°) | 83.809(2) | Goodness-of-fit on F2 | 1.002 |
| β/(°) | 89.595(2) | Final R indices [I>2σ(I)] | R1=0.0694, wR2=0.1758 |
| γ/(°) | 65.708(2) | R indices(all data) | R1=0.1965, wR2=0.2323 |
| V/nm3 | 2.3039(7) |
| Product | δ |
|---|---|
| 1 | δH: 7.39—7.34(m, 5H, Ar—H), 5.46—5.41(m, 1H, CH), 4.62—4.45(m, 2H, CH2), 3.01(br, 1H, OH). δC: 138.1, 128.7, 128.5, 125.9, 81.1, 70.7 |
| 2 | δH: 7.23—7.11(m, 4H, Ar—H), 5.46—5.40(m, 1H, CH), 4.64—4.46(m, 2H, CH2), 2.74(br, 1H, OH), 2.34(s, 3H, CH3). δC: 138.6, 134.9, 129.4, 125.6, 80.9, 70.6, 20.9 |
| 3 | δH: 7.46 —7.15(m, 4H, Ar—H), 5.70(m, 1H, CH), 4.48—4.39(m, 2H, CH2), 2.88(br, 1H, OH), 2.33(s, 3H, CH3). δC: 136.2, 134.4, 130.9, 128.7, 126.8, 125.7, 80.3, 68.0, 21.2 |
| 4 | δH: 7.29(d, J=8.6 Hz, 2H, Ar—H), 6.86(d, J=8.6 Hz, 2H, Ar—H), 5.36—5.32(m, 1H, CH), 4.57—4.36(m, 2H, CH2), 3.74(s, 3H, CH3), 2.72(br, 1H,OH). δC: 159.6, 130.0, 126.9, 114.0, 80.9, 70.3, 55.0 |
| 5 | δH: 7.40—7.21(m, 4H, Ar—H), 5.59—5.54(m, 1H, CH), 4.65—4.56(m, 2H, CH2), 3.84(s, 3H, OCH3), 3.17(br, 1H, OH). δC: 155.8, 129.7, 127.0, 125.9, 121.0, 110.4, 79.8, 67.6, 55.3 |
| 6 | δH: 8.29(d, J=8.7 Hz, 2H, Ar—H ), 7.64(d, J=8.7 Hz, 2H, Ar—H ), 5.62—5.60(m, 1H, CH), 4.63—4.54(m, 2H, CH2), 3.16(br, 1H, OH). δC: 147.7, 144.7, 126.6, 123.9, 80.3, 69.6 |
| 7 | δH: 8.05(d, J=8.1 Hz, 1H, Ar—H), 7.93(d, J=7.7 Hz, 1H, Ar—H), 7.73(m, 1H, Ar—H), 7.53(m, 1H, Ar—H), 6.02—5.99(m, 1H, CH), 4.77—4.54(m, 2H, CH2), 3.21(br, 1H, OH). δC: 147.5, 135.0, 134.8, 130.0, 129.2, 125.4, 80.7, 67.3 |
| 8 | δH: 7.58—7.55(d, J=8.3 Hz, 2H, Ar—H ), 7.31—7.29(d, J=8.3 Hz, 2H, Ar—H), 5.46—5.44(m, 1H, CH), 4.60—4.48(m, 2H, CH2), 2.94(br, 1H, OH). δC: 136.8, 131.8, 127.3, 122.6, 80.6, 70.0 |
| 9 | δH: 7.60(d, J=7.5 Hz, 1H, Ar—H), 7.45(d, J=8.0 Hz, 1H, Ar—H), 7.34—7.19(m, 2H, Ar—H), 5.78—5.73(m, 1H, CH), 4.63—4.47(m, 2H, CH2), 3.22(br, 1H, OH). δC: 136.8, 132.7, 130.0, 127.9, 127.7, 121.5, 79.6, 70.1 |
Table 2 1H NMR(CDCl3, δH) and 13C NMR(CDCl3, δC) data of the products
| Product | δ |
|---|---|
| 1 | δH: 7.39—7.34(m, 5H, Ar—H), 5.46—5.41(m, 1H, CH), 4.62—4.45(m, 2H, CH2), 3.01(br, 1H, OH). δC: 138.1, 128.7, 128.5, 125.9, 81.1, 70.7 |
| 2 | δH: 7.23—7.11(m, 4H, Ar—H), 5.46—5.40(m, 1H, CH), 4.64—4.46(m, 2H, CH2), 2.74(br, 1H, OH), 2.34(s, 3H, CH3). δC: 138.6, 134.9, 129.4, 125.6, 80.9, 70.6, 20.9 |
| 3 | δH: 7.46 —7.15(m, 4H, Ar—H), 5.70(m, 1H, CH), 4.48—4.39(m, 2H, CH2), 2.88(br, 1H, OH), 2.33(s, 3H, CH3). δC: 136.2, 134.4, 130.9, 128.7, 126.8, 125.7, 80.3, 68.0, 21.2 |
| 4 | δH: 7.29(d, J=8.6 Hz, 2H, Ar—H), 6.86(d, J=8.6 Hz, 2H, Ar—H), 5.36—5.32(m, 1H, CH), 4.57—4.36(m, 2H, CH2), 3.74(s, 3H, CH3), 2.72(br, 1H,OH). δC: 159.6, 130.0, 126.9, 114.0, 80.9, 70.3, 55.0 |
| 5 | δH: 7.40—7.21(m, 4H, Ar—H), 5.59—5.54(m, 1H, CH), 4.65—4.56(m, 2H, CH2), 3.84(s, 3H, OCH3), 3.17(br, 1H, OH). δC: 155.8, 129.7, 127.0, 125.9, 121.0, 110.4, 79.8, 67.6, 55.3 |
| 6 | δH: 8.29(d, J=8.7 Hz, 2H, Ar—H ), 7.64(d, J=8.7 Hz, 2H, Ar—H ), 5.62—5.60(m, 1H, CH), 4.63—4.54(m, 2H, CH2), 3.16(br, 1H, OH). δC: 147.7, 144.7, 126.6, 123.9, 80.3, 69.6 |
| 7 | δH: 8.05(d, J=8.1 Hz, 1H, Ar—H), 7.93(d, J=7.7 Hz, 1H, Ar—H), 7.73(m, 1H, Ar—H), 7.53(m, 1H, Ar—H), 6.02—5.99(m, 1H, CH), 4.77—4.54(m, 2H, CH2), 3.21(br, 1H, OH). δC: 147.5, 135.0, 134.8, 130.0, 129.2, 125.4, 80.7, 67.3 |
| 8 | δH: 7.58—7.55(d, J=8.3 Hz, 2H, Ar—H ), 7.31—7.29(d, J=8.3 Hz, 2H, Ar—H), 5.46—5.44(m, 1H, CH), 4.60—4.48(m, 2H, CH2), 2.94(br, 1H, OH). δC: 136.8, 131.8, 127.3, 122.6, 80.6, 70.0 |
| 9 | δH: 7.60(d, J=7.5 Hz, 1H, Ar—H), 7.45(d, J=8.0 Hz, 1H, Ar—H), 7.34—7.19(m, 2H, Ar—H), 5.78—5.73(m, 1H, CH), 4.63—4.47(m, 2H, CH2), 3.22(br, 1H, OH). δC: 136.8, 132.7, 130.0, 127.9, 127.7, 121.5, 79.6, 70.1 |
| Zn1—O6 | 0.1980(6) | Zn1—O7 | 0.1956(6) | Zn1—O8 | 0.1962(6) |
|---|---|---|---|---|---|
| Zn1—O9 | 0.1980(6) | Zn2—N4 | 0.2080(7) | Zn2—N1 | 0.2085(7) |
| Zn2—N3 | 0.2138(8) | Zn2—O1 | 0.2165(7) | Zn2—N2 | 0.2193(7) |
| Zn2—O2 | 0.2302(6) | Zn3—N8 | 0.2067(7) | Zn3—N5 | 0.2079(8) |
| Zn3—N6 | 0.2140(9) | Zn3—N7 | 0.2145(8) | Zn3—O4 | 0.2229(7) |
| Zn3—O3 | 0.2260(7) | ||||
| O7—Zn1—O8 | 116.5(3) | O7—Zn1—O6 | 113.9(3) | O8—Zn1—O6 | 99.8(3) |
| O7—Zn1—O9 | 97.0(3) | O8—Zn1—O9 | 114.4(3) | O6—Zn1—O9 | 116.3(3) |
| N4—Zn2—N1 | 111.0(3) | N4—Zn2—O1 | 105.5(3) | N3—Zn2—N2 | 176.3(3) |
| N1—Zn2—O2 | 88.0(3) | O1—Zn2—O2 | 57.7(2) | N8—Zn3—N5 | 105.3(3) |
| N6—Zn3—N7 | 167.5(4) | N5—Zn3—O4 | 106.1(3) | N8—Zn3—O3 | 93.2(3) |
| O4—Zn3—O3 | 58.2(2) |
Table 3 Selected bond lengths(nm) and bond angles(°) of the complex
| Zn1—O6 | 0.1980(6) | Zn1—O7 | 0.1956(6) | Zn1—O8 | 0.1962(6) |
|---|---|---|---|---|---|
| Zn1—O9 | 0.1980(6) | Zn2—N4 | 0.2080(7) | Zn2—N1 | 0.2085(7) |
| Zn2—N3 | 0.2138(8) | Zn2—O1 | 0.2165(7) | Zn2—N2 | 0.2193(7) |
| Zn2—O2 | 0.2302(6) | Zn3—N8 | 0.2067(7) | Zn3—N5 | 0.2079(8) |
| Zn3—N6 | 0.2140(9) | Zn3—N7 | 0.2145(8) | Zn3—O4 | 0.2229(7) |
| Zn3—O3 | 0.2260(7) | ||||
| O7—Zn1—O8 | 116.5(3) | O7—Zn1—O6 | 113.9(3) | O8—Zn1—O6 | 99.8(3) |
| O7—Zn1—O9 | 97.0(3) | O8—Zn1—O9 | 114.4(3) | O6—Zn1—O9 | 116.3(3) |
| N4—Zn2—N1 | 111.0(3) | N4—Zn2—O1 | 105.5(3) | N3—Zn2—N2 | 176.3(3) |
| N1—Zn2—O2 | 88.0(3) | O1—Zn2—O2 | 57.7(2) | N8—Zn3—N5 | 105.3(3) |
| N6—Zn3—N7 | 167.5(4) | N5—Zn3—O4 | 106.1(3) | N8—Zn3—O3 | 93.2(3) |
| O4—Zn3—O3 | 58.2(2) |
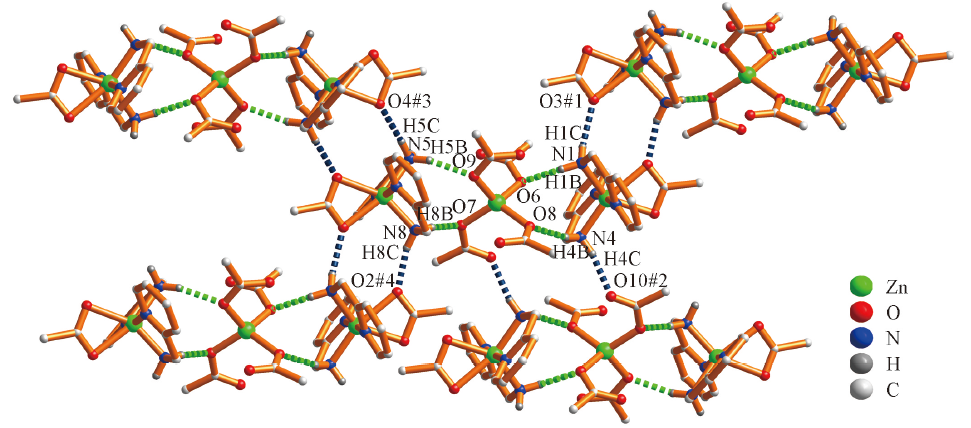
Fig.2 2D network structure of the complex via hydrogen bonds^Symmetry code: #1: x, -1+y, z; #2: 2-x, -y, 1-z; #3: 2-x, 1-y, -z; #4: x, 1+y, z. H atoms except amino are omitted for clarity.
| D—H…A | d(D—H)/nm | d(H…A)/nm | d(D…A)/nm | ∠(DHA)/(°) |
|---|---|---|---|---|
| N1—H1B…O6 | 0.0900 | 0.2360 | 0.3095 | 139.00 |
| N1— H1C…O3#1 | 0.0900 | 0.2180 | 0.3008 | 152.00 |
| N4—H4B…O8 | 0.0900 | 0.2110 | 0.2998 | 167.00 |
| N4—H4C…O10#2 | 0.0900 | 0.2080 | 0.2941 | 161.00 |
| N5—H5B…O9 | 0.0900 | 0.2100 | 0.2961 | 160.00 |
| N5—H5C…O4#3 | 0.0900 | 0.2050 | 0.2953 | 176.00 |
| N8—H8B…O7 | 0.0900 | 0.2090 | 0.2975 | 166.00 |
| N8—H8C…O2#4 | 0.0900 | 0.2010 | 0.2870 | 161.00 |
Table 4 Hydrogen bonds of the complex*
| D—H…A | d(D—H)/nm | d(H…A)/nm | d(D…A)/nm | ∠(DHA)/(°) |
|---|---|---|---|---|
| N1—H1B…O6 | 0.0900 | 0.2360 | 0.3095 | 139.00 |
| N1— H1C…O3#1 | 0.0900 | 0.2180 | 0.3008 | 152.00 |
| N4—H4B…O8 | 0.0900 | 0.2110 | 0.2998 | 167.00 |
| N4—H4C…O10#2 | 0.0900 | 0.2080 | 0.2941 | 161.00 |
| N5—H5B…O9 | 0.0900 | 0.2100 | 0.2961 | 160.00 |
| N5—H5C…O4#3 | 0.0900 | 0.2050 | 0.2953 | 176.00 |
| N8—H8B…O7 | 0.0900 | 0.2090 | 0.2975 | 166.00 |
| N8—H8C…O2#4 | 0.0900 | 0.2010 | 0.2870 | 161.00 |
| Entry | Catalyst loading (molar fraction, %) | Solvent | Time/h | Yield(%) | Entry | Catalyst loading (molar fraction, %) | Solvent | Time/h | Yield(%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | Methanol | 20 | 21.0 | 8 | 9 | Methanol | 15 | 68.0 |
| 2 | 6 | Methanol | 20 | 51.5 | 9 | 9 | Methanol | 25 | 83.5 |
| 3 | 9 | Methanol | 20 | 83.5 | 10 | 9 | THF | 20 | 80.5 |
| 4 | 12 | Methanol | 20 | 84.5 | 11 | 9 | CH2Cl2 | 20 | 71.0 |
| 5 | 15 | Methanol | 20 | 84.0 | 12 | 9 | Et2O | 20 | 77.5 |
| 6 | 9 | Methanol | 5 | 0 | 13 | 9 | Zn(OAc)2 | 20 | 15 |
| 7 | 9 | Methanol | 10 | 15.0 | 14 | 9 | AMPy | 20 | 0 |
Table 5 Optimization of the reaction conditions of nitromethane to benzaldehyde
| Entry | Catalyst loading (molar fraction, %) | Solvent | Time/h | Yield(%) | Entry | Catalyst loading (molar fraction, %) | Solvent | Time/h | Yield(%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | Methanol | 20 | 21.0 | 8 | 9 | Methanol | 15 | 68.0 |
| 2 | 6 | Methanol | 20 | 51.5 | 9 | 9 | Methanol | 25 | 83.5 |
| 3 | 9 | Methanol | 20 | 83.5 | 10 | 9 | THF | 20 | 80.5 |
| 4 | 12 | Methanol | 20 | 84.5 | 11 | 9 | CH2Cl2 | 20 | 71.0 |
| 5 | 15 | Methanol | 20 | 84.0 | 12 | 9 | Et2O | 20 | 77.5 |
| 6 | 9 | Methanol | 5 | 0 | 13 | 9 | Zn(OAc)2 | 20 | 15 |
| 7 | 9 | Methanol | 10 | 15.0 | 14 | 9 | AMPy | 20 | 0 |
| Entry | Substrate | Yield(%) | Entry | Substrate | Yield(%) |
|---|---|---|---|---|---|
| 1 | C6H5CHO | 83.5 | 6 | p-NO2C6H4CHO | 90.5 |
| 2 | p-CH3C6H4CHO | 79.0 | 7 | o-NO2C6H4CHO | 87.5 |
| 3 | o-CH3C6H4CHO | 72.5 | 8 | p-BrC6H4CHO | 85.0 |
| 4 | p-MeOC6H4CHO | 69.0 | 9 | o-BrC6H4CHO | 86.5 |
| 5 | o-MeOC6H4CHO | 59.0 |
Table 6 Catalytic reactions of nitromethane to different aromatic aldehydes
| Entry | Substrate | Yield(%) | Entry | Substrate | Yield(%) |
|---|---|---|---|---|---|
| 1 | C6H5CHO | 83.5 | 6 | p-NO2C6H4CHO | 90.5 |
| 2 | p-CH3C6H4CHO | 79.0 | 7 | o-NO2C6H4CHO | 87.5 |
| 3 | o-CH3C6H4CHO | 72.5 | 8 | p-BrC6H4CHO | 85.0 |
| 4 | p-MeOC6H4CHO | 69.0 | 9 | o-BrC6H4CHO | 86.5 |
| 5 | o-MeOC6H4CHO | 59.0 |
| [1] | Boruwa J., Gogoi N., Saikia P. P., Barua N. C., Tetrahedron: Asymmetry, 2006, 17(24), 3315—3326 |
| [2] | Peera A. A., Tomlinson I. A., Tetrahedron Lett., 2012, 53(26), 3322—3324 |
| [3] | Paintner F. F., Allmendinger L., Bauschke G., Klemann P., Organic Lett., 2005, 7(7), 1423—1426 |
| [4] | Sasidharan M., Bhaumik A., Journal of Molecular Catalysis A: Chemical, 2013, 367, 1—6 |
| [5] | Luzzio F. A., Tetrahedron, 2001, 57(6), 915—945 |
| [6] | Nenajdenko V. G., Druzhinin S. V., Balenkova E. S., Tetrahedron Letters, 2005, 46(51), 8853—8855 |
| [7] | Kogami Y., Nakajima T., Ikeno T., Yamada T.,Synthesis, 2004, (12), 1947—1950 |
| [8] | Kowalczyk R., Kwiatkowski P., Skarzewski J., Jurczak J., Journal of Organic Chemistry, 2009, 74(2), 753—756 |
| [9] | Xu B., Zhang Y. H., Tian H., Yao Y. M., Journal of Lanzhou University, 2013, 49(1), 138—142 |
| (徐宾, 张永红, 田花, 姚英明.兰州大学学报, 2013,49(1), 138—142) | |
| [10] | Luo M., Tang H. M., Li Q. R., Sun J., Yang S. Z., Li X. L., Journal of Chemical Sciences, 2009, 121(4), 435—440 |
| [11] | Nguyen Q. T., Jeong J. H., Polyhedron, 2006, 25(8), 1787—1790 |
| [12] | Zhang Y. B., Zhou H. L., He C. T., Xue W., Zhang J. P., Chen X. M., Chem. J. Chinese Universities, 2011, 32(3), 497—502 |
| (章跃标, 周浩龙, 何纯挺, 薛玮, 张杰鹏, 陈小明.高等学校化学学报, 2011,32(3), 497—502) | |
| [13] | Enoki O., Imaoka T., Yamamoto K., Organic Letters, 2003, 5(14), 2547—2549 |
| [14] | Huang Q. M., Wang S. W., Li Q., Pan W., Deng P. X., Zhou H., Pan Z. Q., Chem. J. Chinese Universities, 2012, 33(4), 732—737 |
| (黄齐茂, 王司卫, 李清, 潘威, 邓鹏星, 周红, 潘志权.高等学校化学学报, 2012,33(4), 732—737) | |
| [15] | Yang Y. Q., Chen Z. M., Kuang Y. F., Chinese J. Inorg. Chem., 2013, 29(1), 185—189 |
| (杨颖群, 陈志敏, 匡云飞.无机化学学报, 2013,29(1), 185—189) | |
| [16] | Anselmo D., Bocokic V., Decortes A., Reek J. N. H., Kleij A. W., Polyhedron, 2012, 32(1), 49—53 |
| [17] | Wang Y., Ren Q. Z., Guo L. T., Hou Z. S., Jiang Z. R., Chem. J. Chinese Universities, 2013, 34(7), 1576—1584 |
| (王颖, 任奇志, 郭琳童, 候宗胜, 蒋宗润.高等学校化学学报, 2013,34(7), 1576—1584) | |
| [18] | Eckert P. K., Vieira I. S., Gessner V. H., Börner J., Strohmann C., Herres-Pawli S., Polyhedron, 2013, 49(1), 151—157 |
| [19] | Liu M., Huang Y. D., Wang Y. M., Tetrahedron: Asymmetry, 2013, 24(12), 736—743 |
| [20] | Xu S. S., Zhou Y. H., Qu J. P., Chin. J. Org. Chem., 2012, 32(6), 1131—1135 |
| (徐珊珊, 周宇涵, 曲景平.有机化学, 2012,32(6), 1131—1135) |
| [1] | ZHENG Anni, JIN Lei, YANG Jiaqiang, WANG Zhaoyun, LI Weiqing, YANG Fangzu, ZHAN Dongping, TIAN Zhongqun. Effects of 5,5-Dimethylhydantoin on Electroless Copper Plating [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220191. |
| [2] | LIU Qingqing, WANG Pu, WANG Yongshuai, ZHAO Man, DONG Huanli. Synthesis and Topochemical Polymerization Study of Naphthalene/perylene Imides Substituted Diacetylene Derivatives [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220091. |
| [3] | SHI Naike, ZHANG Ya, SANSON Andrea, WANG Lei, CHEN Jun. Uniaxial Negative Thermal Expansion and Mechanism in Zn(NCN) [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220124. |
| [4] | ZHOU Yonghui, LI Yao, WU Yuxuan, TIAN Jing, XU Longquan, FEI Xu. Synthesis of A Novel Photoluminescence Self-healing Hydrogel [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210606. |
| [5] | ZHU Haotian, JIN Meixiu, TANG Wensi, SU Fang, LI Yangguang. Properties of Transition Metal-biimidazole-Dawson-type Tungstophosphate Hybrid Compounds as Supports for Enzyme Immobilization [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220328. |
| [6] | ZHANG Taiwen, GUO Jun, ZHANG Dan, YUAN Changmei, QIU Shuangyan. Synthesis, Characterization and Catalytic Oxidation Iodine Ion Performance of trz-Cl-Cu-PMo12 [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220215. |
| [7] | ZHOU Yonghui, HUANG Rujun, YAN Jianyang, LI Yajun, QIU Huanhuan, YANG Jinxuan, ZHENG Youxuan. Synthesis and Electroluminescence Properties of Two Iridium(Ⅲ) Complexes with Nitrogen Heterocycle Structures [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210415. |
| [8] | WEI Chuangyu, CHEN Yanli, JIANG Jianzhuang. Fabrication of Electrochemical Sensor for Dopamine and Uric Acid Based on a Novel Dimeric Phthalocyanine-involved Quintuple-decker Modified Indium Tin Oxide Electrode [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210582. |
| [9] | YUE Shengli, WU Guangbao, LI Xing, LI Kang, HUANG Gaosheng, TANG Yi, ZHOU Huiqiong. Research Progress of Quasi-two-dimensional Perovskite Solar Cells [J]. Chem. J. Chinese Universities, 2021, 42(6): 1648. |
| [10] | WANG Le, QIN Liulei, LIU Yang, REN Li, XU Huiting, LIU Zunqi. Synthesis, Structure and Dielectric Properties of One-dimensional Chain Hydrogen Glycine Supramolecular Compound [(Gly)2+(18-crown-6)2(MnCl4)2‒] [J]. Chem. J. Chinese Universities, 2021, 42(3): 691. |
| [11] | ZHANG Huidong, GU Panpan, ZHANG Fang, DU Mingxu, YE Kaiqi, LIU Yu. Design and Electroluminescence Properties of Narrow-spectrum Phosphorescent Complexes [J]. Chem. J. Chinese Universities, 2021, 42(12): 3571. |
| [12] | LI Xiaolei, SUN Yunjiao, TANG Ying, WANG Changsheng. Rapid and Accurate Calculation of the Three⁃body Interaction Strength in the Hydrogen⁃bonded Complexes of Alcohols or Deoxyribose with Water [J]. Chem. J. Chinese Universities, 2021, 42(12): 3664. |
| [13] | DING Xin, SHI Hongdong, LIU Yangzhong. Using Human Serum Albumin Nanoparticle as Carrier for co-Delivery of Ru(Ⅲ) Complex and All-trans-retinoic Acid and Its Application for Anti-Metastasis Therapy [J]. Chem. J. Chinese Universities, 2021, 42(10): 3040. |
| [14] | YANG Ju, SU Lijiao, LI Canhua, LU Jiajia, YANG Junli, GU Jie, YANG Li, YANG Lijuan. Host-guest Complexation Behavior of Nardosinone and Water-soluble Phosphate Salt Pillar[6]arene [J]. Chem. J. Chinese Universities, 2021, 42(10): 3099. |
| [15] | HUANG Yunshuai, YANG Ni, WU Zehong, WU Si. Ruthenium Complexes with Photoresponsive Coordination Bonds for Light-controlled Surface Functions † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1174. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||