

高等学校化学学报 ›› 2023, Vol. 44 ›› Issue (1): 20220665.doi: 10.7503/cjcu20220665
杨霁野1,孙大吟1,王妍1,谷安祺1,叶一兰1,丁书江2( ),杨振忠1(
),杨振忠1( )
)
收稿日期:2022-10-10
出版日期:2023-01-10
发布日期:2022-11-26
基金资助:
YANG Jiye1, SUN Dayin1, WANG Yan1, GU Anqi1, YE Yilan1, DING Shujiang2( ), YANG Zhenzhong1(
), YANG Zhenzhong1( )
)
Received:2022-10-10
Online:2023-01-10
Published:2022-11-26
Contact:
DING Shujiang, YANG Zhenzhong
E-mail:dingsj@mail.xjtu.edu.cn;yangzhenzhong@tsinghua.edu.cn
Supported by:摘要:
中空结构材料作为一类新兴功能材料, 具有可调空腔、 高比例活性表面及强化的物质传递等特性; 当多组分及功能被整合与分区时, 可实现中空结构材料的非对称结构(Janus)的拓扑演化. 本文重点介绍若干典型中空结构材料, 包括Janus中空材料的模板合成方法进展及中空结构材料在催化、 储能、 油/水分离与药物递送等领域的潜在应用, 并展望了中空结构材料的未来发展趋势.
中图分类号:
TrendMD:
杨霁野, 孙大吟, 王妍, 谷安祺, 叶一兰, 丁书江, 杨振忠. 若干典型中空结构材料的模板合成与应用进展. 高等学校化学学报, 2023, 44(1): 20220665.
YANG Jiye, SUN Dayin, WANG Yan, GU Anqi, YE Yilan, DING Shujiang, YANG Zhenzhong. Progresses in Template Synthesis and Applications of Hollow Materials. Chem. J. Chinese Universities, 2023, 44(1): 20220665.
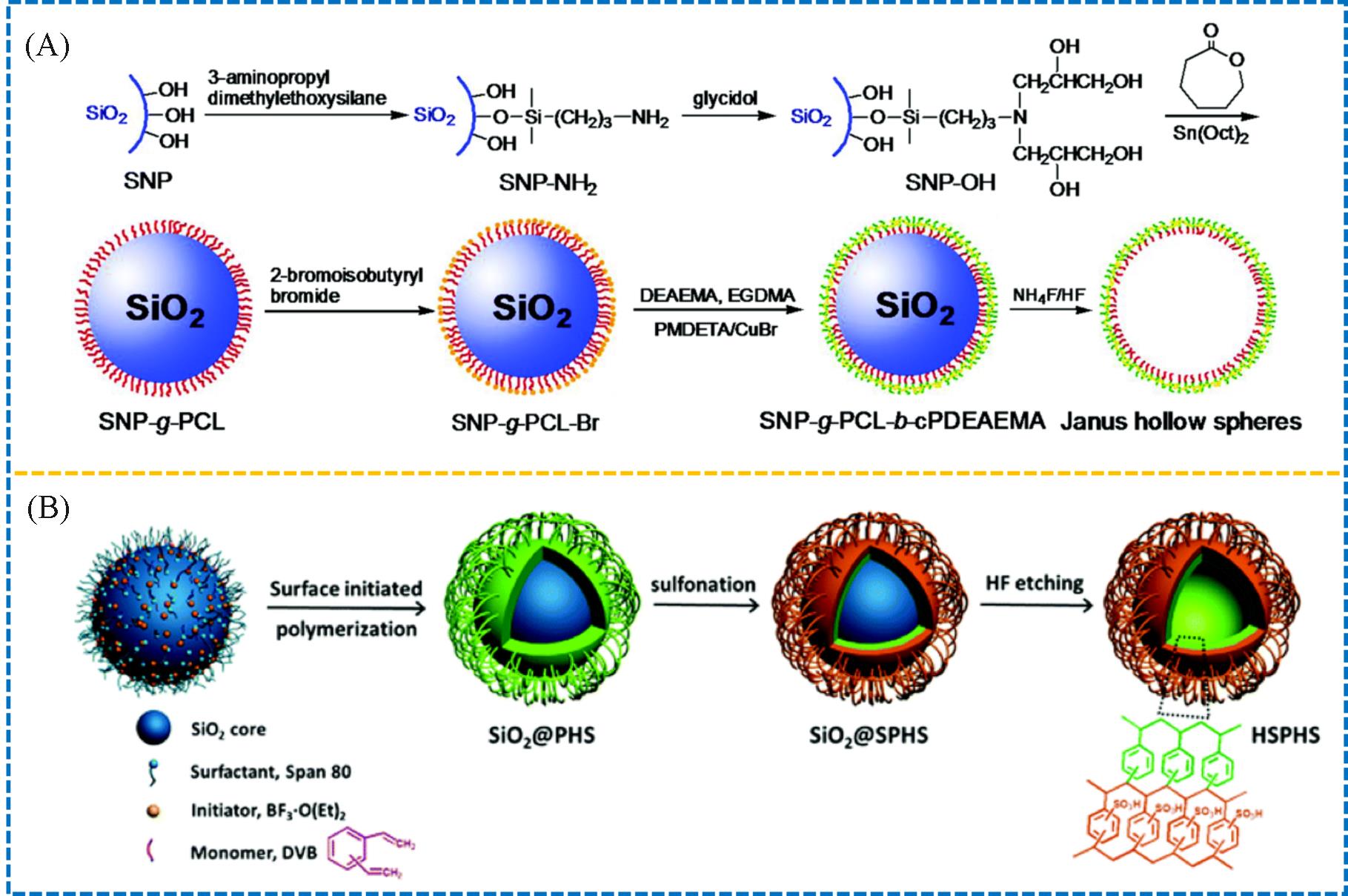
Fig.1 Synthesis of the Janus hollow spheres of PCL⁃PDEAEMA(A)[13] and polystyrene⁃sulfonated polystyrene gel(B)[14](A) Copyright 2015, the Royal Society of Chemistry; (B) Copyright 2019, the Royal Society of Chemistry.
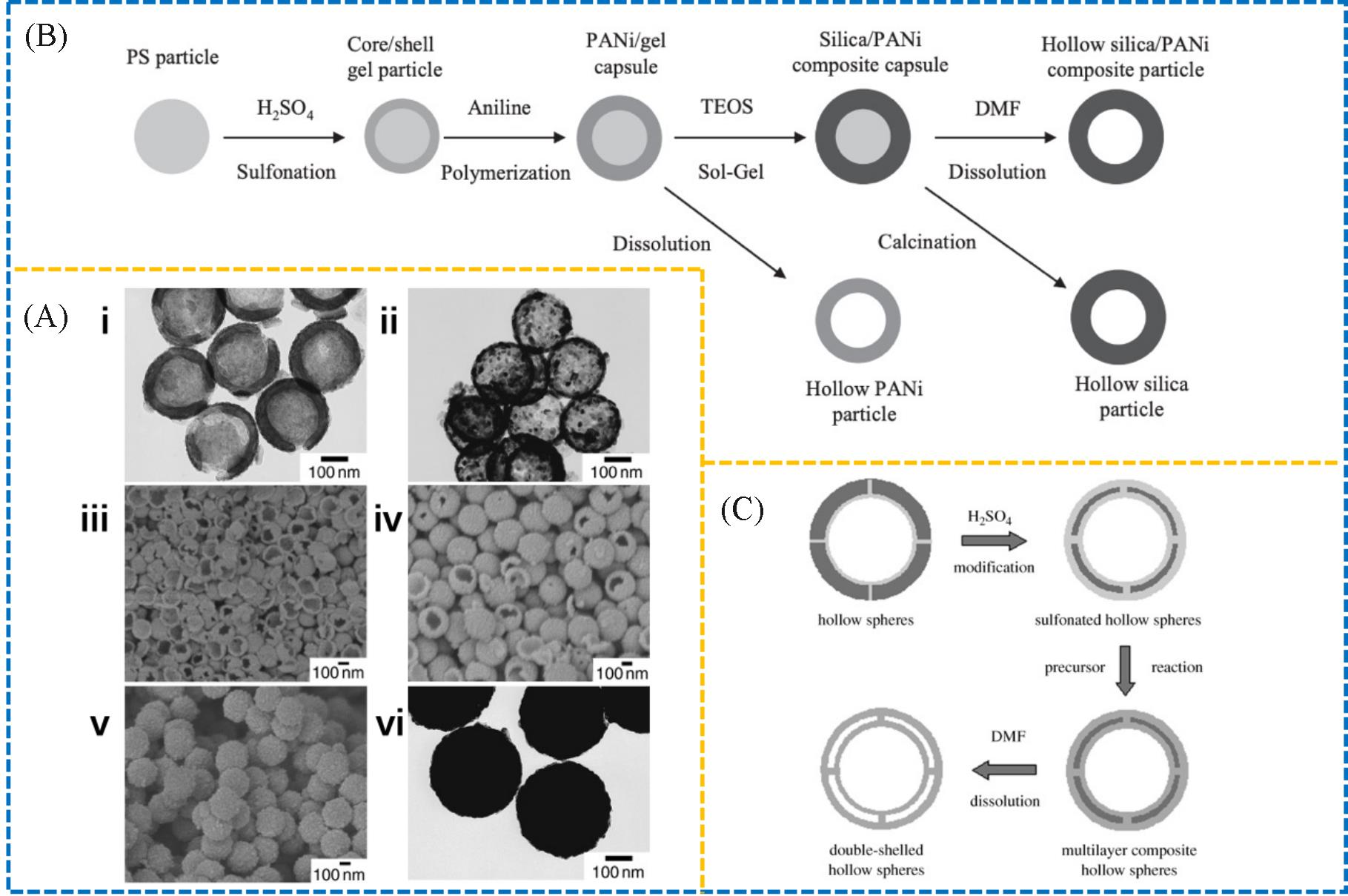
Fig.2 SEM and TEM images of the representative titania hollow spheres with tunable shell thickness and cavity size(A)[15], preparation of the composite capsules and the corresponding hollow spheres of PANi and silica(B)[16] and formation of the double⁃shelled hollow sphere against sPS⁃PS⁃sPS sandwiched hollow sphere template(C)[17](A) Copyright 2003, John Wiley and Sons; (B) Copyright 2003, John Wiley and Sons; (C) Copyright 2005, John Wiley and Sons.
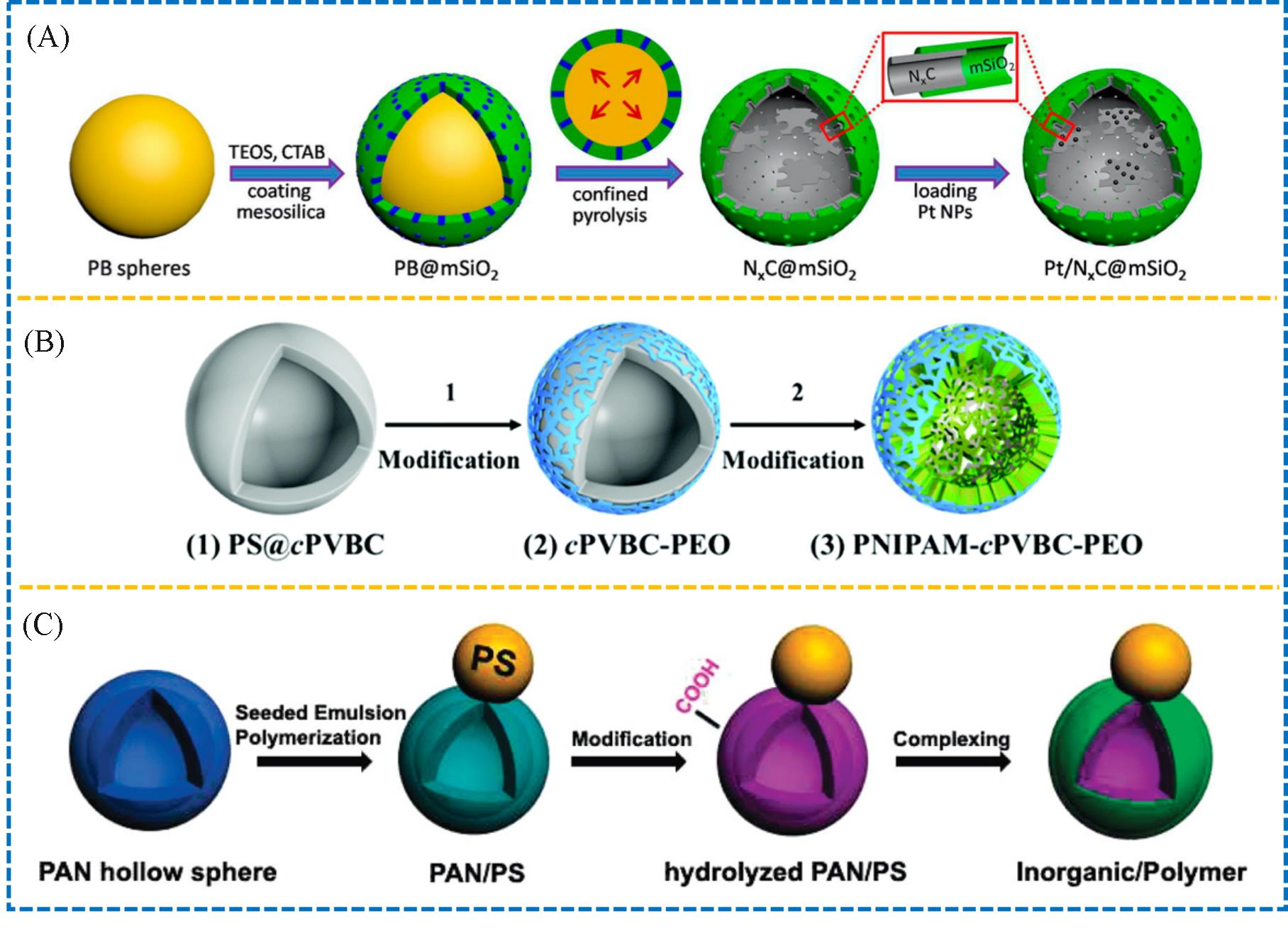
Fig.3 Schematic preparation of a multifunctional Janus hollow sphere of N x C@mSiO2(A)[18], illustrative synthesis of the thermally responsive Janus porous hollow sphere of PNIPAM⁃cPVBC⁃PEO(B)[19], and schematic synthesis of the snowman⁃like hollow sphere with dually Janus features(C)[20](A) Copyright 2018, American Chemical Society; (B) Copyright 2020, the Royal Society of Chemistry; (C) Copyright 2010, American Chemical Society.
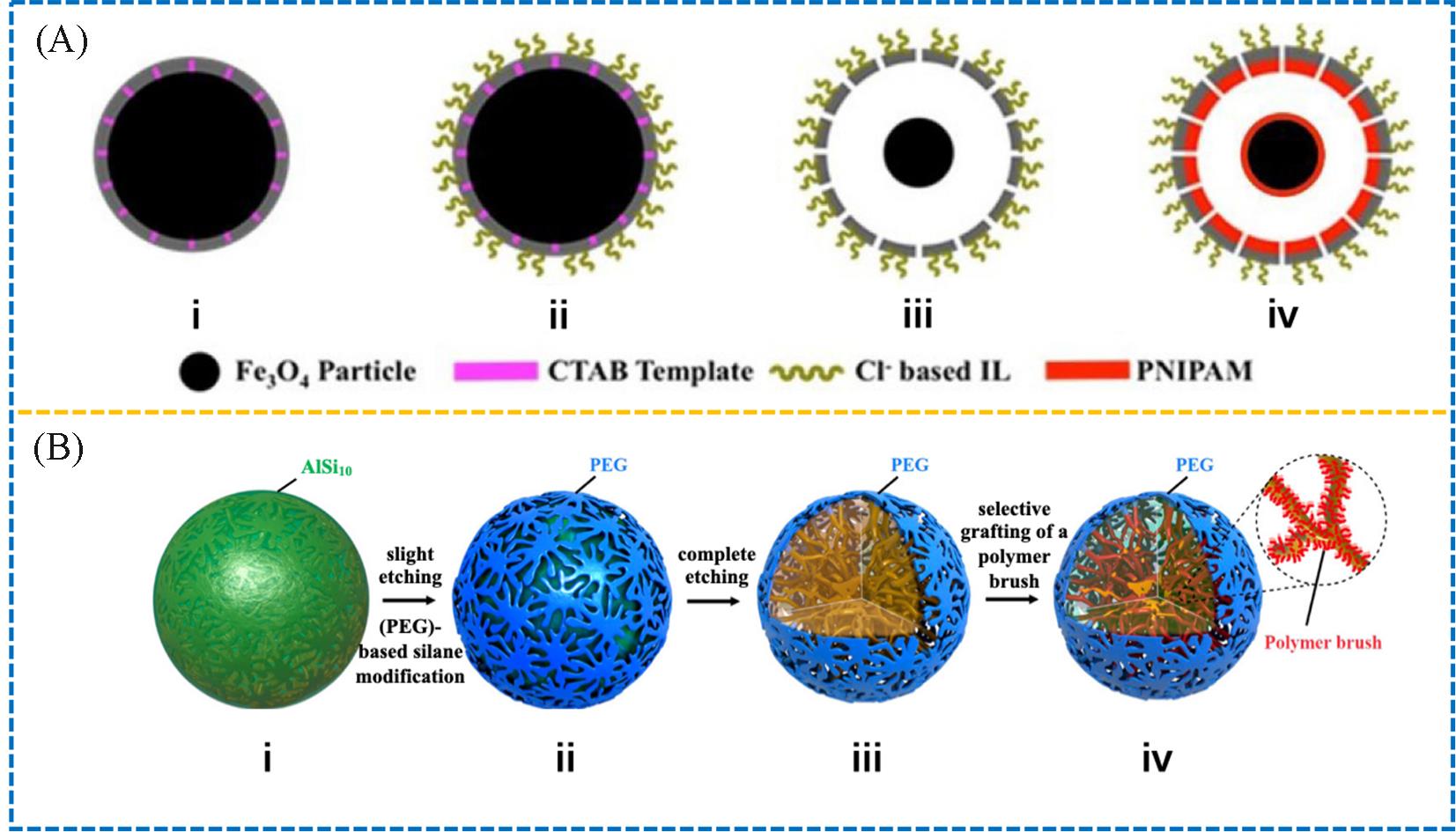
Fig.4 Illustrative synthesis of the IL/PNIPAM magnetic Janus hollow sphere(A)[24] and illustrative synthesis of the Janus coral⁃like porous sphere by stepwise de⁃alloying of the AlSi10 alloy sphere and subsequential modification(B)[25](A) Copyright 2019, John Wiley and Sons; (B) Copyright 2016, American Chemical Society.
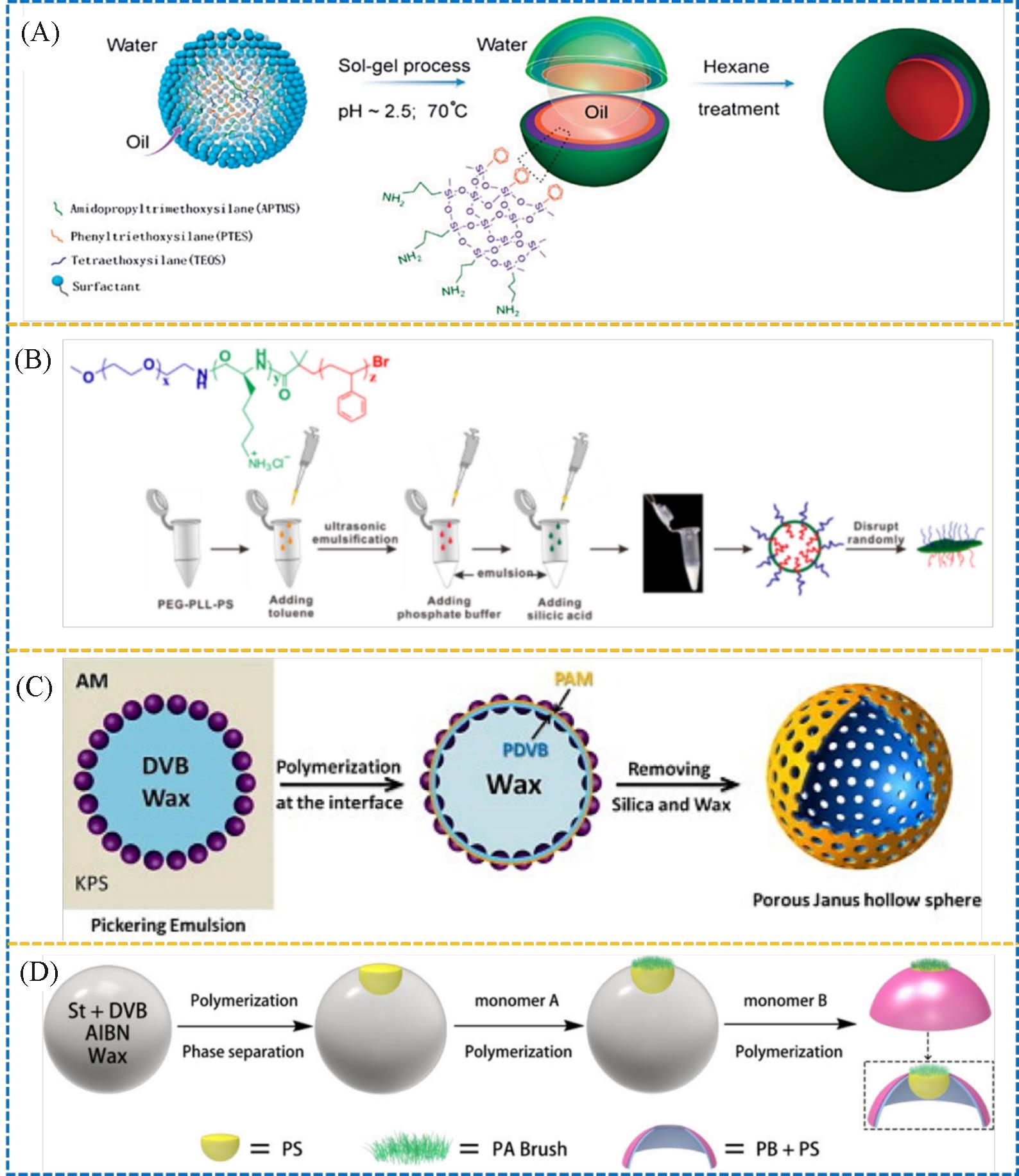
Fig.5 Schematic synthesis of the Janus hollow sphere by emulsion interfacial self⁃organized sol⁃gel process(A)[34], schematic illustration of the biomimetic synthesis of JHS(B)[35], schematic synthesis of the Janus macroporous hollow sphere by using Pickering emulsion template(C)[36] and schematic synthesis of the jellyfish⁃like Janus hollow sphere by two⁃step emulsion interfacial polymerization(D)[37](A) Copyright 2011, the Royal Society of Chemistry; (B) Copyright 2015, American Chemical Society; (C) Copyright 2012, Elsevier; (D) Copyright 2020, American Chemical Society.
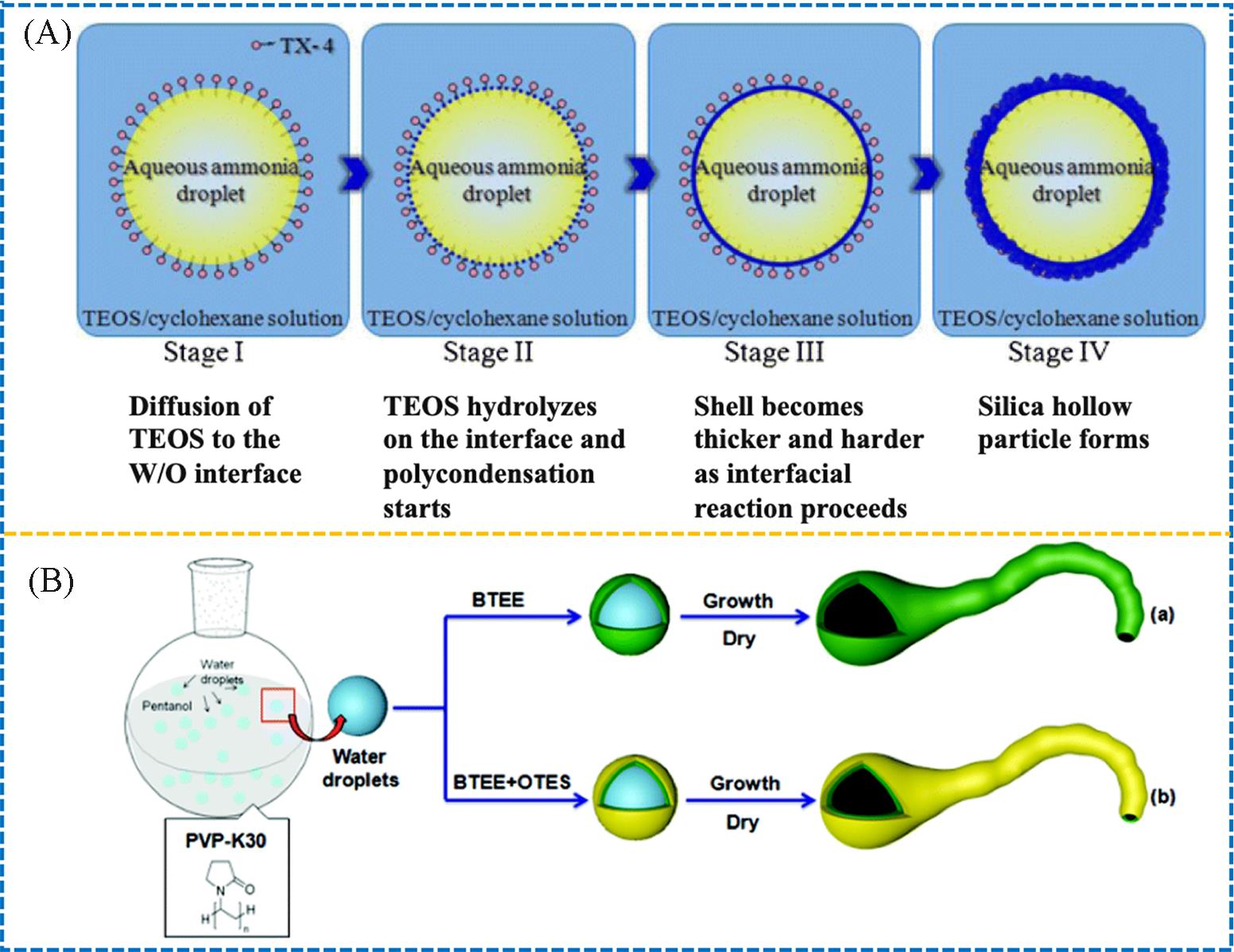
Fig.6 Schematic synthesis of the silica hollow sphere by W/O inverse emulsion template(A)[38] and schematic formation of the tadpole⁃like nanotube and the corresponding Janus one(B)[39](A) Copyright 2013, Springer Nature; (B) Copyright 2021, the Royal Society of Chemistry.
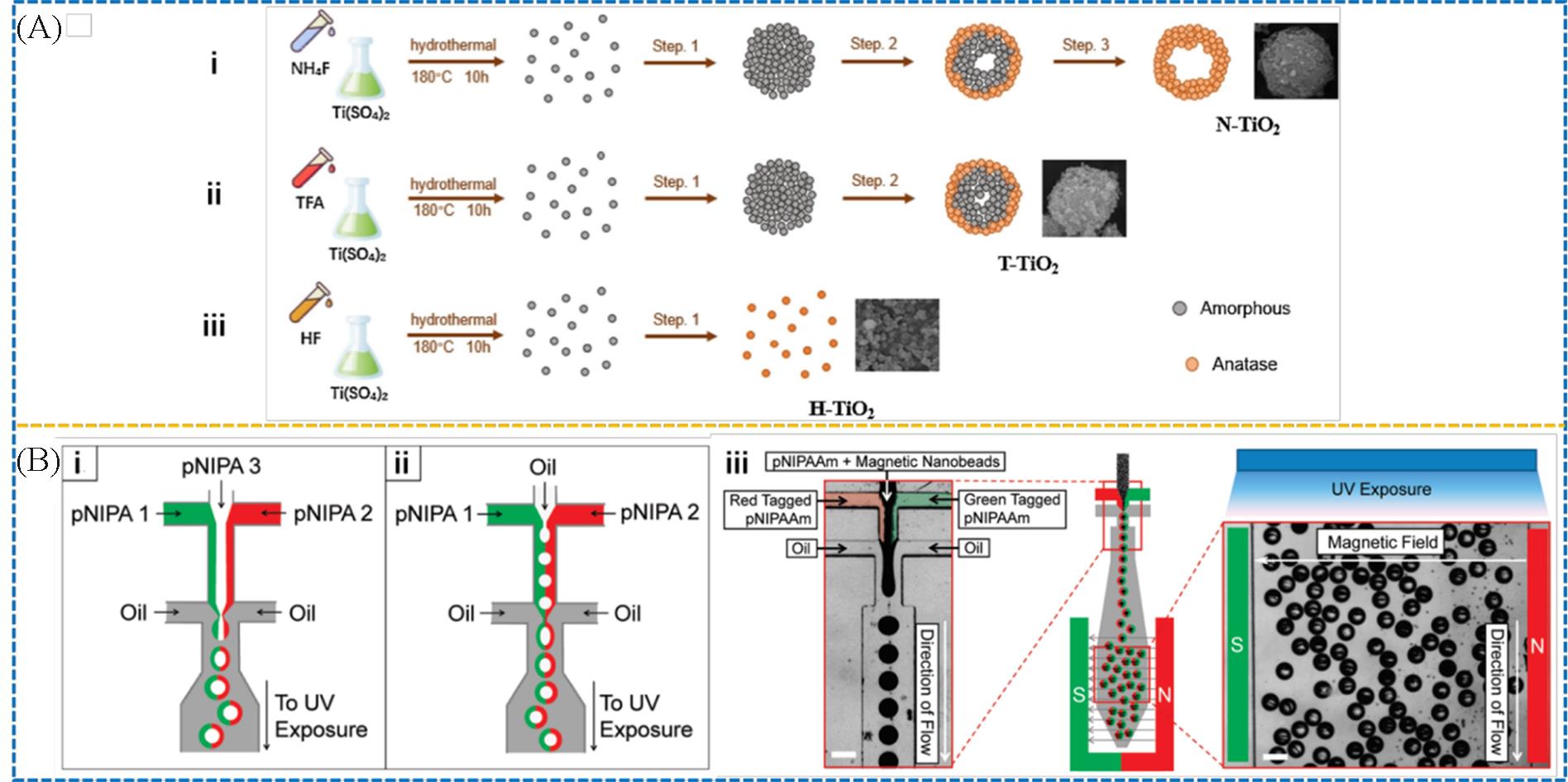
Fig.7 Schematic fluoride⁃induced self⁃transformation of TiO2 toward hollow structures in different ion environments(A)[45] and formation of Janus microgels and microshells(B)[56](A) Copyright 2021, Elsevier; (B) Copyright 2010, American Chemical Society.
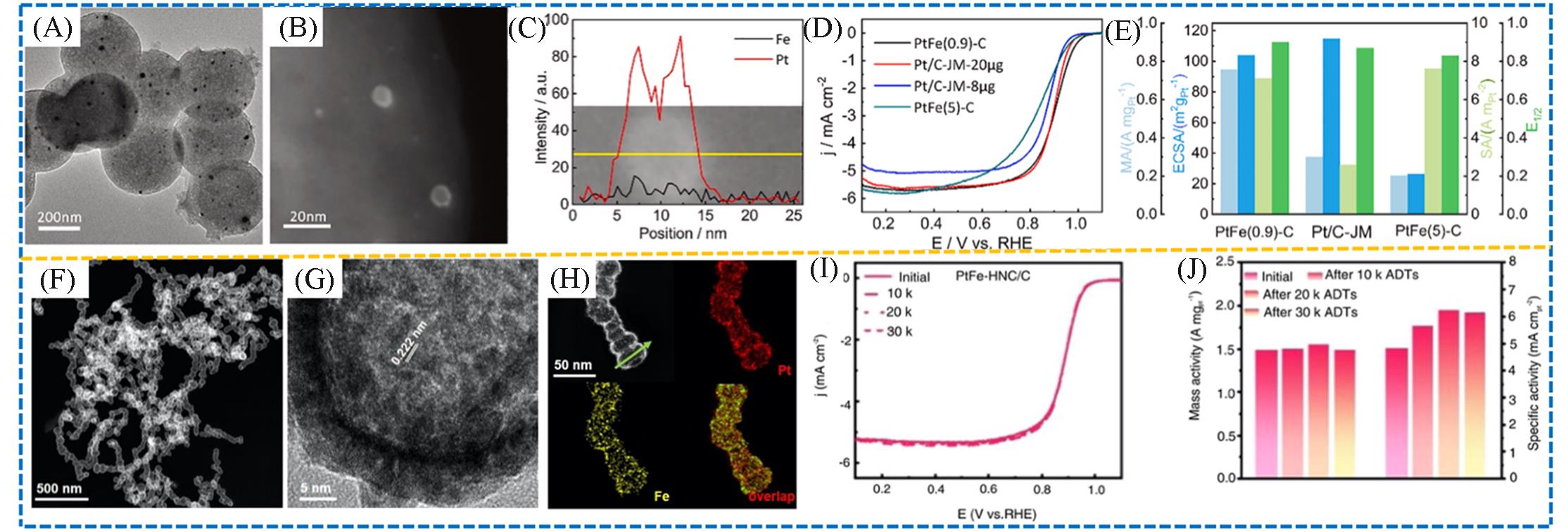
Fig.8 TEM image(A), STEM image(B) and line scanning of the PtFe(0.9)⁃C(C), LSV polarization curves of the sample(D), mass activity, specific activity, and electrochemical surface area measured at 0.9 V and half⁃wave potential versus commercial Pt/C(E)[60], TEM and EDS images of the PtFe‐HNC(F—H), LSV curves(I) and MA and SA results before and after durability test of the PtFe‐HNC/C(J)[61]
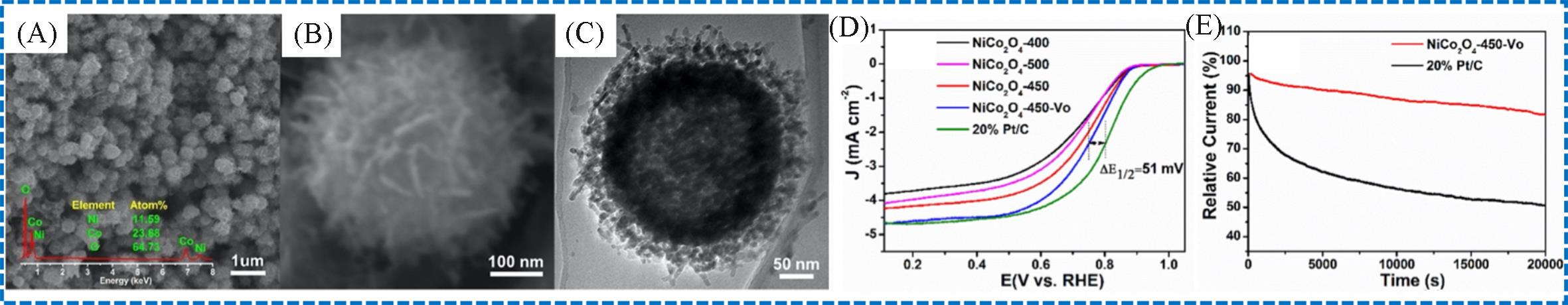
Fig.9 SEM images of the NiCo2O4⁃450⁃Vo at two magnifications(A, B), TEM image of the NiCo2O4⁃ 450⁃Vo(C), LSVs of different catalysts in O2⁃saturated 0.1 mol/L KOH(D), chronoamperometric curves(normalized to initial current) of the NiCo2O4⁃450⁃Vo and Pt/C at 0.6 V vs. RHE(E)[62]
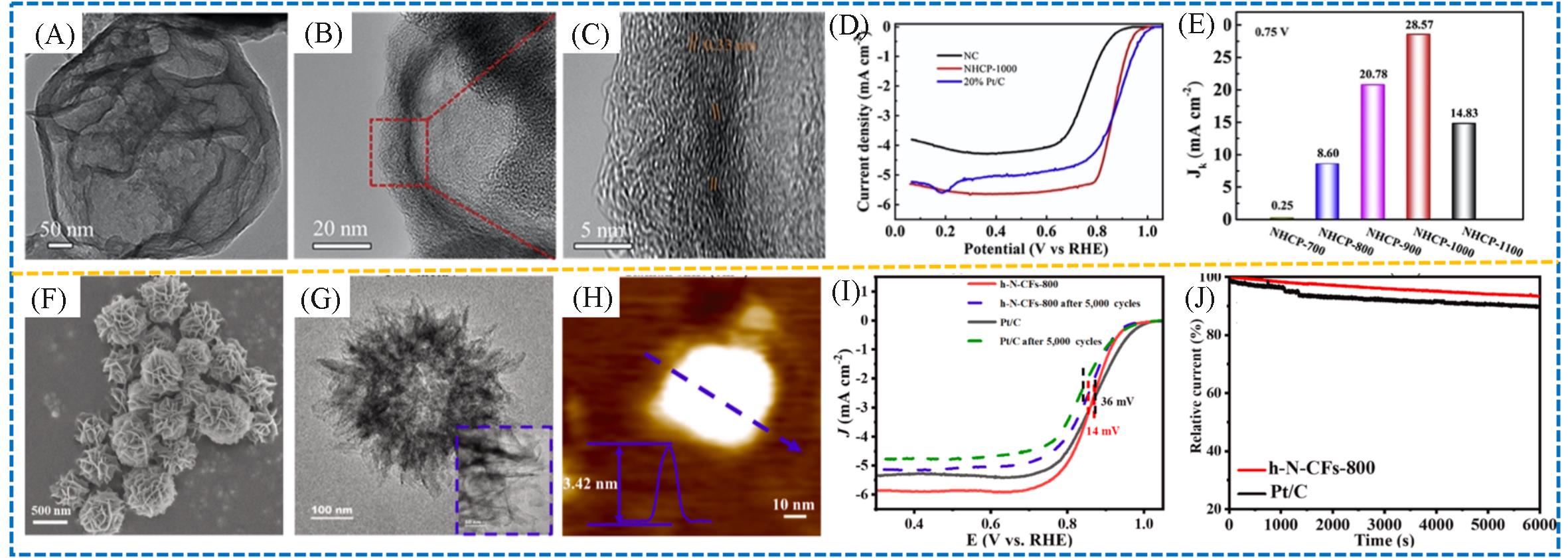
Fig.10 TEM image(A) and HR⁃TEM images(B, C) of the NHCP⁃1000, ORR polarization curves of the NC, NHCP⁃1000 and commercial 20%(mass fraction) Pt/C in O2 saturated 0.1 mol/L KOH solution at 1600 r/min with a scanning rate of 10 mV/s(D), Jk at 0.75 V versus the RHE of NHCP at varied pyrolysis temperature(E)[63], SEM(F), TEM(G) and AFM(H) images of the h⁃N⁃CF⁃800, durability of the catalysts before and after 5000 cycles(I), chronoamperometric test of the h⁃N⁃CF⁃800 in O2 purged 0.1 mol/L KOH electrolyte(J)[64]
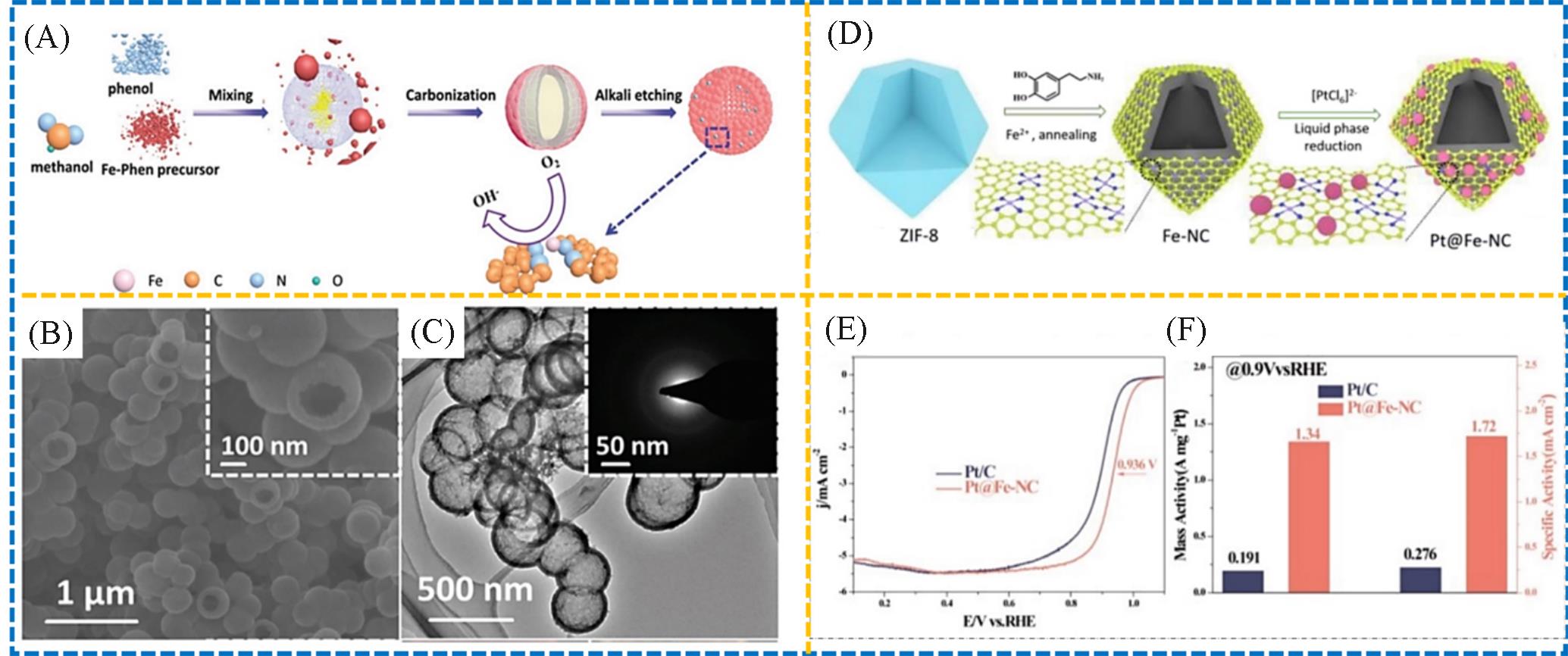
Fig.11 Schematic synthesis(A), SEM(B) and TEM(C) images of the hollow Fe⁃N/C⁃800[65], synthesis of the Pt@Fe⁃NC catalyst(D), LSV plots of the Pt/C and Pt@Fe⁃NC(E), mass activity and specific activities of the Pt/C and Pt@Fe⁃NC at 0.9 V versus RHE(F)[66]
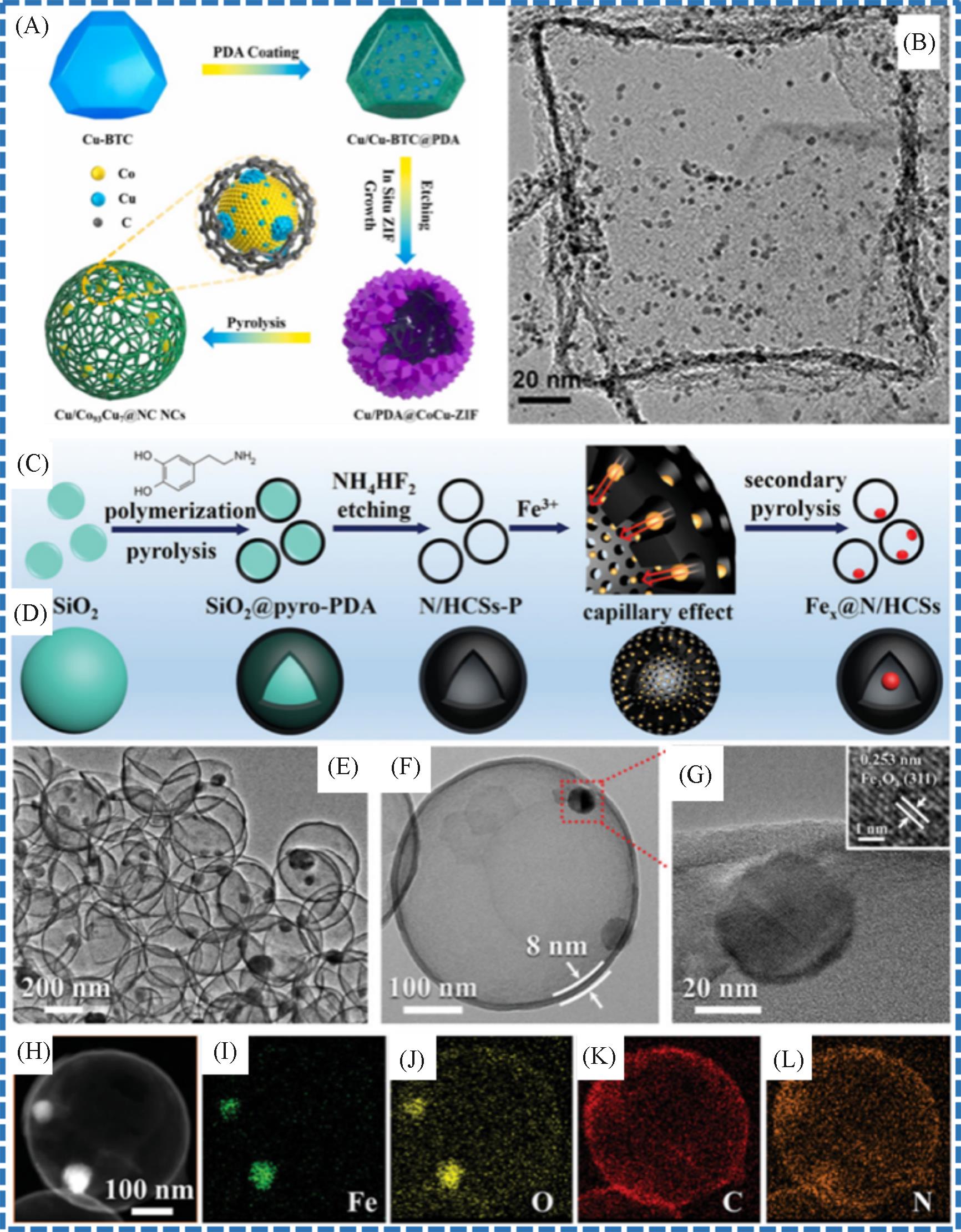
Fig.12 Synthesis of the Cu/Co93Cu7@NC NCs catalyst(A)[67], TEM image of the Pt@GB(B)[68], synthesis of the Fe x @N/HCS(C, D), TEM images(E, F), HR⁃TEM image(G) and elemental mapping(H—I) of the Fe20@N/HCS catalyst[69](A) Copyright 2022, Elsevier; (B) Copyright 2014, John Wiley and Sons; (C—I) Copyright 2020, John Wiley and Sons.
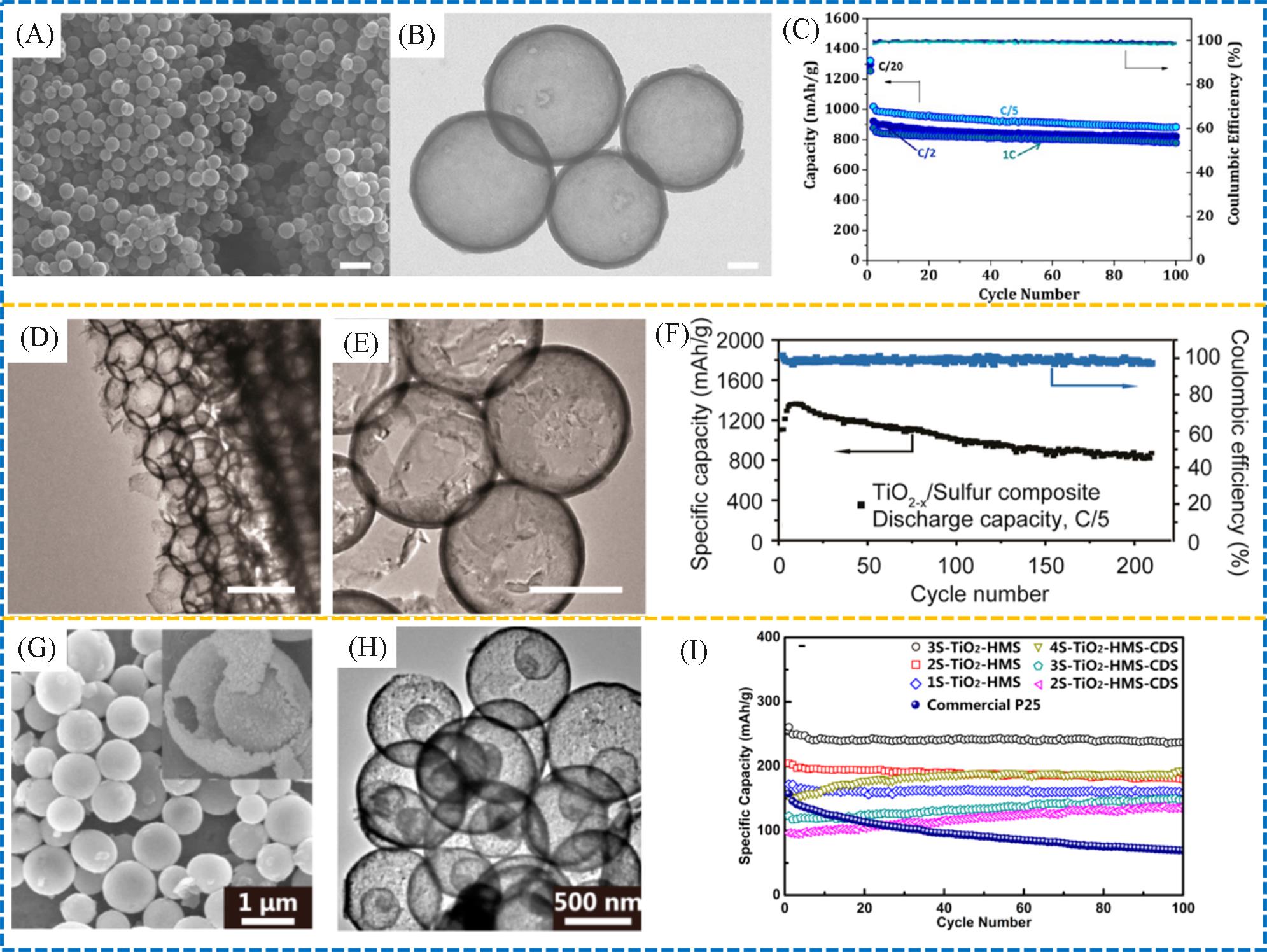
Fig.13 SEM(A)and STEM(B) images of PCNSs, rate capability of p⁃PCNS⁃M⁃70 electrode over 100 cycles at various current rates(C)[72], TEM (D) and zoom⁃in images(E) of the reduced hollow TiO2 nanospheres, cycling performance of TiO2-x /sulfur composite cathode at a current rate of C/5(F)[73], SEM(G) and TEM(H) micrographs of 3S⁃TiO2⁃HMS, cycling performance at the current rate of 1 C between 1.0 and 3.0 V(I)[75]
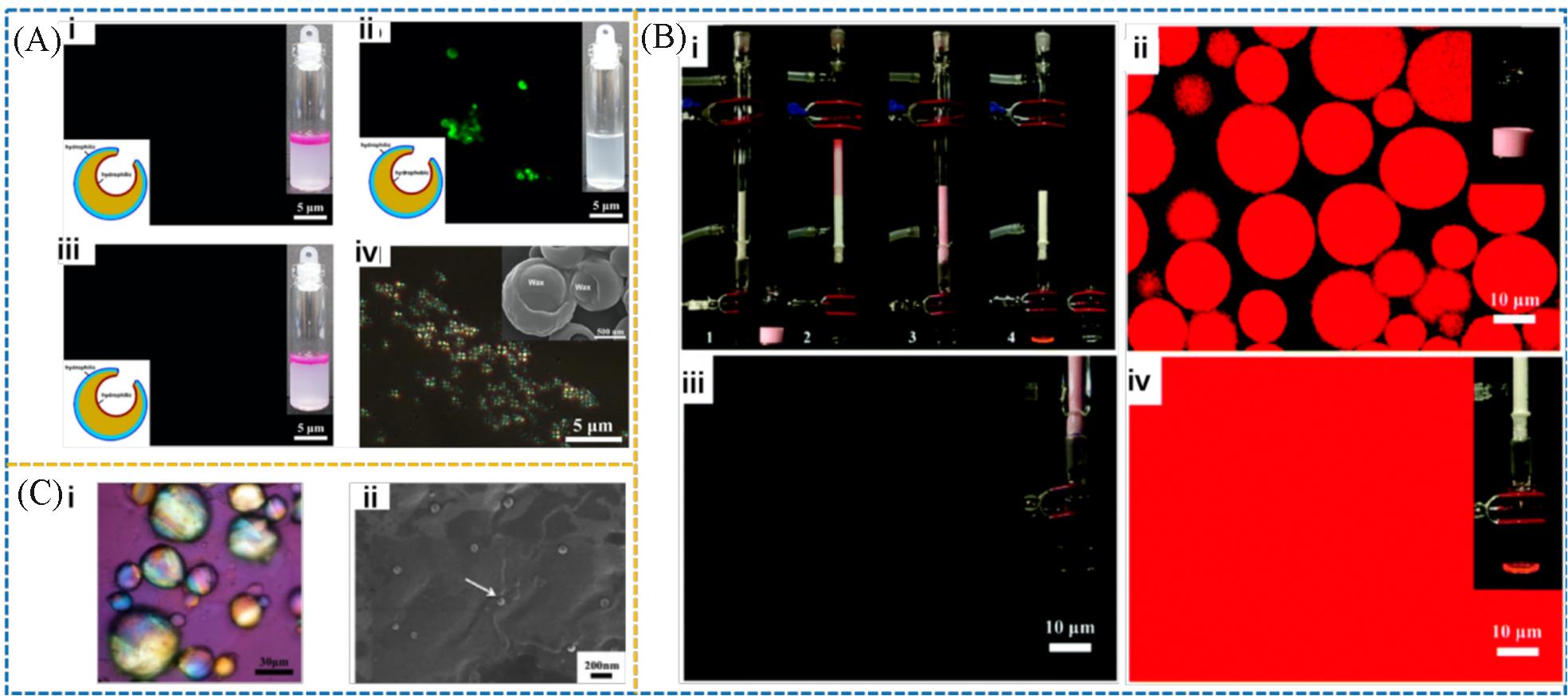
Fig.14 Fluorescence microscopy images of the PDEAEMA⁃EP@silica⁃PEO Janus hollow sphere in the n⁃hexane/water mixture at varied pHs(A)[77], separation of n⁃hexane/water emulsion by thermoresponsive PNIPAM⁃cPVBC⁃PEO Janus cage column(B)[19] and adsorption of paraffin/toluene and modified lipophilic silica particles by PAM/PDVB Janus cage(C)[36](A) (i) pH=4, (ii) pH=10, (iii) pH=4, n-hexane dyed with dil-C18; the insert schematics in i-iii illustrate the different wettability of interior/exterior surface in Janus hollow sphere, (iv) polarizing optical image; inset is the SEM image of the Janus hollow sphere after loading wax inside the cavity at pH=10. (B)(i) (1) The PNIPAM-cPVBC-PEO Janus cage column at 40 ℃, (2) feeding the SDS stabilized n-hexane/water emulsion into the column, (3) n-hexane was captured inside the Janus cage while water eluted, (4) n-hexane was released from the cage when cooling down to 25 ℃; (ii) CLSM image of the SDS stabilized n-hexane/water emulsion; (iii) CLSM image of the eluted water phase; (iv) CLSM image of n-hexane released from the cage at 25 ℃. (C)(i) Fluorescence microscopy image of the PAM/PDVB Janus cages with paraffin/toluene preferentially absorbed inside the cavity; (ii) SEM image of the PAM/PDVB Janus cage after the mixture of paraffin wax (Tm: 52 ℃) and the modified lipophilic silica particles were absorbed inside the cavity, the microsphere was sliced in order to observe the internal structure.(A) Copyright 2022, American Chemical Society; (B) Copyright 2020, the Royal Society of Chemistry; (C) Copyright 2012, Elsevier.
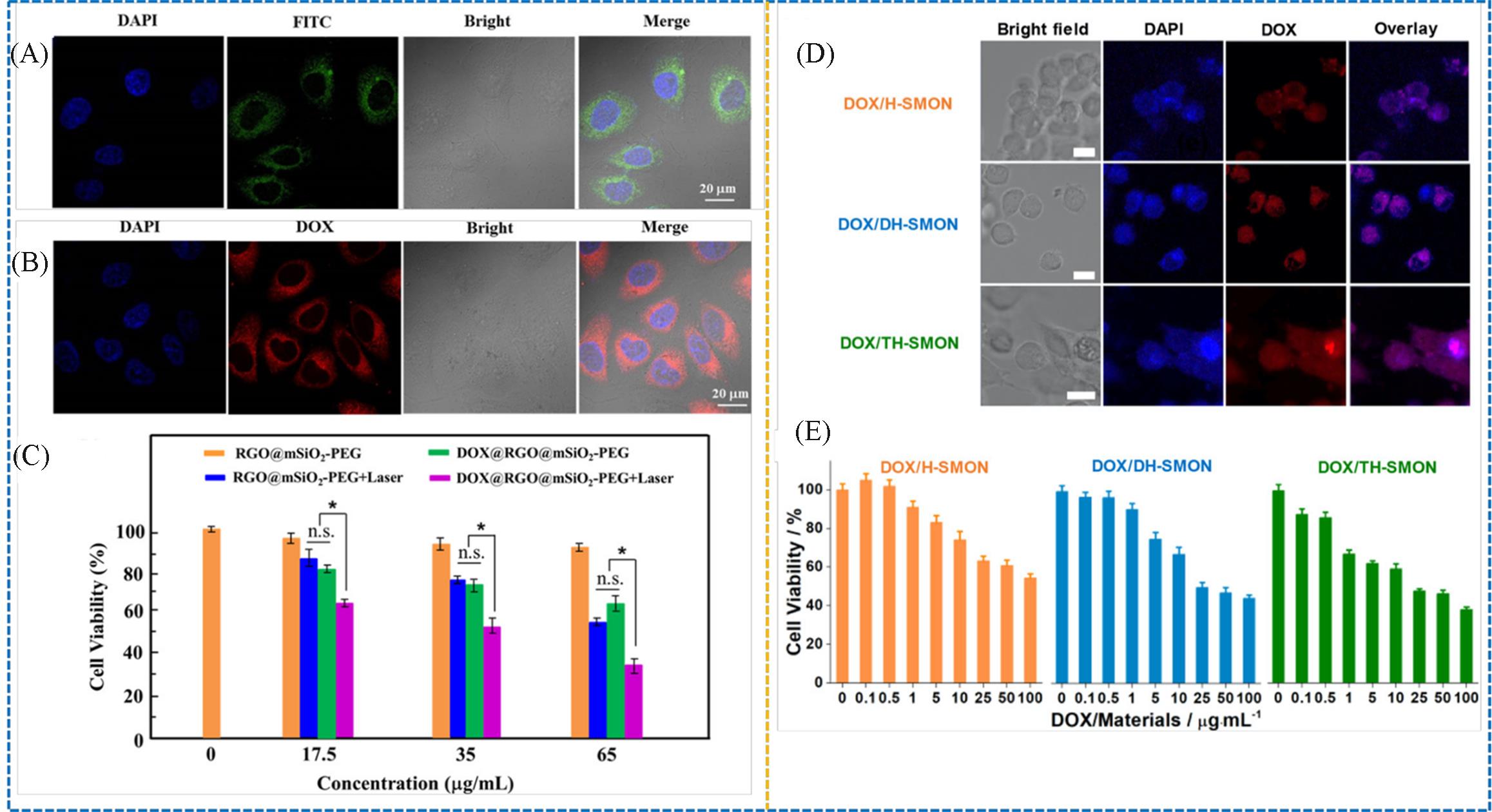
Fig.15 CLSM images of the cells after uptaking the FITC⁃labeled Janus nanocage(A), CLSM images of the HeLa cells after uptaking the DOX@RGO@mSiO2⁃PEG Janus nanocage for 12 h(B), viabilities of the HeLa cells under different treatments(C)[30], CLSM images(D) and cell viabilities(E) after treating the cancer cells with DOX⁃loaded multi⁃shelled hollow spheres[79](A) The nuclei displayed blue after staining with DAPI, the FITC-labeled Janus nanocages displayed green; (B) the nucleus displayed blue after staining with DAPI, the RGO@mSiO2-PEG Janus nanocages displayed red after loading DOX; (D) CLSM images after treating the cancer cells(MCF7 cell line) with the DOX-loaded H-SMON(single-shelled hollow microporous organic network), DH-SMON(double-shelled hollow microporous organic network), and TH-SMON(triple-shelled hollow microporous organic network) for 1 h(scale bar=10 μm); (E) cell viabilities of the cancer cells(MCF7 cell line) after the treatment with DOX-loaded H-SMON, DH-SMON, and TH-SMON for 2 d.
| 1 | Kim H., Lah M. S., Dalton Trans., 2017, 46(19), 6146—6158 |
| 2 | Zhang S., Li D. H., Chen S., Yang X. F., Zhao X. L., Zhao Q. S., Komarneni S., Yang D. J., J. Mater. Chem. A, 2017, 5(24), 12453—12461 |
| 3 | Huang M., Mi K., Zhang J. H., Liu H. L., Yu T. T., Yuan A. H., Kong, Q. H., Xiong S. L., J. Mater. Chem. A, 2017, 5(1), 266—274 |
| 4 | Yang F., Jin W., Lin Y. C., Wang C., Lut H., Tan Y. Z., J. Lightwave Technol., 2017, 35(16), 3413—3424 |
| 5 | Yazdi A., Mercoçi F, Bastús N. G., Imaz I., Puntes V., Maspoch D., Catal. Sci. Technol., 2016, 6(24), 8388—8391 |
| 6 | Gennes P. G., Science, 1992, 256, 495—497 |
| 7 | Ojani R., Valiollahi R., Raoof J. B., Energy, 2014, 74, 871—876 |
| 8 | Zhang J., Cao Y. D., Wang C. A., Ran R., ACS Appl. Mater. Interfaces, 2016, 8(13), 8670—8677 |
| 9 | Zhang Y. X., Yu Y. F., Liang K. H., Liu L., Shen Z. M., Chen A. B., New J. Chem., 2019, 43(27), 10899—10905 |
| 10 | Rashid M., Bui T. T., Hoang A. T., Seong G. H., Lim D. W., Kim Y. S., Bull. Korean Chem. Soc., 2015, 36(12), 2940—2943 |
| 11 | Zhang S. H., Ren J. Y., Zhang Y., Peng H. C., Chen S., Yang F., Cao Y., Org. Electron., 2020, 77, 105497 |
| 12 | Chen H., Wu Y. K., Duan J. J., Zhan R. M., Wang W., Wang M. Q., Chen Y. M., Xu M. W., Bao S. J., ACS Appl. Mater. Interfaces, 2019, 11(45), 42197—42205 |
| 13 | Zhao Z. G., Zhu F. Y., Qu X. Z., Wu Q. H., Wang Q., Zhang G. L., Liang F. X., Polym. Chem., 2015, 6(22), 4144—4153 |
| 14 | Wu G., Zhap L. L., Liang H. Z., Yan Y. H., Tan H., Mater. Chem. Front., 2019, 3(5), 922—930 |
| 15 | Yang Z. Z., Niu Z. Z., Lu Y. Y., Hu Z. B., Han C. C., Angew. Chem. Int. Ed., 2003, 42(17), 1943—1945 |
| 16 | Niu Z. W., Yang Z. Z., Hu Z. B., Lu Y. F., Han C. C., Adv. Funct. Mater., 2003, 13(12), 949—954 |
| 17 | Yang M., Ma J., Zhang C. L., Yang Z. Z., Lu Y. F., Angew. Chem. Int. Ed., 2005, 44(41), 6727—6730 |
| 18 | Dai J. Y., Zou H. B., Shi Z. Q., Yang H. Q., Wang R. W., Zhang Z. T., Qiu S. L., ACS Appl. Mater. Interfaces, 2018, 10(39), 33474—33483 |
| 19 | Zhang L. L., Shi S. Y., Zhang G. L., Song X. M., Sun D. Y., Liang F. X., Yang Z. Z., Chem. Commun., 2020, 56(72), 10497—10500 |
| 20 | Tang C., Zhang C. L., Liu J. G., Qu X. Z., Li J. L., Yang Z. Z., Macromolecules, 2010, 43(11), 5114—5120 |
| 21 | Hou H. B., Yu D. M., Tian Q., Hu G. H., Langmuir, 2014, 30(7), 1741—1747 |
| 22 | Chen G. Z., Desinan S., Rosei R., Rosei F., Ma D. L., Chem. Commun., 2012, 48(64), 8009—8011 |
| 23 | Guo S. J., Dong S. J., Wang E. K., Chem. Eur. J., 2008, 14(15), 4689—4695 |
| 24 | Si Y., Ji X. Y., Liang F. X., Jiang B. Y., Yang Z. Z., Chem. Asian J., 2019, 14(11), 1917—1920 |
| 25 | Zhang H., Wang Q., Jiang B. Y., Liang F. X., Yang Z. Z., ACS Appl. Mater. Interfaces, 2016, 8(48), 33250—33255 |
| 26 | Li S., Feng J. Q., Kou X. C., Zhao Y. J., Ma X. B., Wang S. P., Energy Fuels, 2018, 32(9), 9692—9700 |
| 27 | Wang Q. H., Zhu Y. X., Xue J., Zhao X. S., Guo Z. P., Wang C., ACS Appl. Mater. Interfaces, 2016, 8(27), 17226—17232 |
| 28 | Purwajanti S., Zhang H. W., Huang X. D., Song H., Yang Y. N., Zhang J., Niu Y. T., Meka A. K., Noonan O., Yu C. Z., ACS Appl. Mater. Interfaces, 2016, 8(38), 25306—25312 |
| 29 | Yuan H., Li J. T., Yang W., Zhuang Z. C., Zhao Y., He L., Xu L., Liao X. B., Zhu R. Q., Mai L. Q., ACS Appl. Mater. Interfaces, 2018, 10(19), 16410—16417 |
| 30 | Tang L., Yang S., Liang F. X., Wang Q., Qu X. Z., Yang Z. Z., ACS Appl. Mater. Interfaces, 2016, 8(19), 12056—12062 |
| 31 | Zhu M. Y., Cheng Y. K., Luo Q., El⁃khateeb M., Zhang Q., Mater. Chem. Front., 2021, 5(6), 2552—2587 |
| 32 | Su Y. C., Zong W., Zhao X. L., Ma S. H., Han X. J., RSC Adv., 2015, 5(100), 82247—82251 |
| 33 | Wang B., Chen J. S., Wu H. B.,Wang Z. Y., Lou X. W., J. Am. Chem. Soc., 2011, 133(43), 17146—17148 |
| 34 | Liang F. X., Liu J. G., Zhang C. L., Qu X. Z., Li J. L., Yang Z. Z., Chem. Commun., 2011, 47(4), 1231—1233 |
| 35 | Sheng L., Chen H., Fu W. X., Li Z. B., Langmuir, 2015, 31(44), 11964—11970 |
| 36 | Li J., Wang Y. H., Zhang C. L., Liang F. X., Qu X. Z., Li J. L., Wang Q., Qiu D., Yang Z. Z., Polymer, 2012, 53(17), 3712—3718 |
| 37 | Shi S. Y., Zhang L. L., Zhang G. L., Song X. M., Sun D. Y., Liang F. X., Yang Z. Z., Macromolecules, 2020, 53(6), 2228—2234 |
| 38 | Hu W. C., Gu H., Wang J. N., Li Y. X., Wang Z. Q., Colloid Polym. Sci., 2013, 291(11), 2697—2704 |
| 39 | Zuo X. Y., Zhang M., Wu Q. H., Li Y. Y., Zhang G. L., Liang F. X., Yang Z. Z., Chem. Commun., 2021, 57(47), 5834—5837 |
| 40 | Liu J., Kim A. Y., Wang L. Q., Palmer B. J., Chen Y. L., Bruinsma P., Bunker B. C., Exarhos G. J., Graff G. L., Rieke P. C., Fryxell G. E., Virden J. W., Tarasevich B. J., Chick L. A., Adv. Colloid Interface Sci., 1996, 69(1—3), 131—180 |
| 41 | Qian H. Y., Tang J., Hossain M. S. A., Bando Y., Wang X., Yamauchi Y., Nanoscale, 2017, 9(42), 16264—16272 |
| 42 | Fan X., Zhang Z., Li G., Rowson N. A., Chem. Eng. Sci., 2004, 59(13), 2639—2645 |
| 43 | Zeng W., Huang Y. P., Xiong Y., Wang N., Xu C., Huang L. S., J. Alloys Compd., 2018, 731, 210—221 |
| 44 | Weng W. S., Lin J,, Du Y. C., Ge X. F., Zhou X. S., Bao J. C., J. Mater. Chem. A, 2018, 6(22), 10168—10175 |
| 45 | Liu Z. Q., Wu J., Wang J. M., Wang R., Liu G. L., Qi Y. F., Xu W. X., Luo G. Q., Xu M. H., Colloids Surf. A-Physicochem. Eng. Asp., 2021, 610, 125702 |
| 46 | Zhang L., Wang H., J. Phys. Chem. C, 2011, 115(38), 18479—18485 |
| 47 | Yec C. C., Zeng H. C., Chem. Mater., 2012, 24(10), 1917—1929 |
| 48 | Yin Y. D., Rioux R. M., Erdonmez C. K., Hughes S., Somorjai G. A., Alivisatos A. P., Science, 2004, 304(5671), 711—714 |
| 49 | Park S. K., Choi J. H., Kang Y. C., Chem. Eng. J., 2018, 354, 327—334 |
| 50 | Tianou H., Wang W. C., Yang X. L., Cao Z. M., Kuang Q., Wang Z., Shan Z. W., Jin M. S., Yin Y. D., Nat. Commun., 2017, 8(1), 1261 |
| 51 | Ji D. X., Fan L., Tao L., Sun Y. J., Li M. G., Yang G. R., Tran T. Q., Ramakrishna S., Guo S. J., Angew. Chem. Int. Ed., 2019, 58(39), 13840—13844 |
| 52 | Sun Y. G., Mayers B. T., Xia Y. N., Nano Lett., 2002, 2(5), 481—485 |
| 53 | Daniel J. R., McCarthy L. A., Ringe E., Boudreau D., RSC Adv., 2018, 9(1), 389—396 |
| 54 | Gruzeł G., Arabasz S., Pawlyta M., Parlinska-Wojtan M., Nanoscale, 2019, 11(12), 5355—5364 |
| 55 | Wang N., Cao P. F., Sun S. J., Ma H. Y., Lin M., Inorg. Chem., 2021, 60(5), 3471—3478 |
| 56 | Seiffert S., Romanowsky M. B., Weitz D. A., Langmuir, 2010, 26(18), 14842—14847 |
| 57 | Zhang Q., Zhang T. R., Ge J. P., Yin Y. D., Nano Lett., 2008, 8(9), 2867—2871 |
| 58 | Han L., Yu X. Y., Lou X. W., Adv. Mater., 2016, 28(23), 4601—4605 |
| 59 | Li B. W. , Zeng H. C., Adv. Mater., 2019, 31(38), 1801104 |
| 60 | Gong L. Y., Liu J., Li Y., Wang X., Luo E. G., Jin Z., Ge J. J., Liu C. P., Xing W., Chem. Eng. J., 2022, 428, 131569 |
| 61 | Rao P., Luo J. M., Li J., Huang W., Sun W., Chen Q., Jia C. M., Liu Z. X., Deng P. L., Shen Y. J., Tian X. L., Carbon Energy, 2022, 4(6), 1003—1010 |
| 62 | Chen S., Huang D. M., Liu D. Y., Sun H. Z., Yan W. J., Wang J. C., Dong M., Tong X. L., Fan W. B., Appl. Catal. B⁃Environ., 2021, 291, 120065 |
| 63 | Yan J., Zheng X. J., Wei C. H., Sun Z. H., Zeng K., Shen L. W., Sun J. W., Rümmeli M. H., Yang R. Z., Carbon, 2021, 171, 320—328 |
| 64 | Li S. M., Wang Y. Z., Ding Y., He Y., Zhang Y. Y., Li S. N., Zhang J., Chen Y., Chem. Eng. J., 2022, 430, 132969 |
| 65 | Wu X. T., Peng L. J., Xiao K., Li N., Liu Z. Q., Chem. Commun., 2021, 57(43), 5258—5261 |
| 66 | Liao W., Zhou S. Y., Wang Z. C., Long J., Chen M. D., Zhou Q., Wang Q. M., J. Mater. Chem. A, 2022, 10(40), 21416—21421 |
| 67 | Ren Y. R., Ye P. C., Chen J. D., Wang H. Y., Ning J. Q., Shen J. L., Zhong Y. J., Hu Y., J. Power Sources, 2022, 545, 231908 |
| 68 | Lv Y. Y., Fang Y., Wu Z. X., Qian X. F., Song Y. F., Che R. C., Asiri A. M., Xia Y. Y., Tu B., Zhao D. Y., Small, 2015, 11(8), 1003—1010 |
| 69 | Wang B., Ye Y. Z., Xu L., Quan Y., Wei W. X., Zhu W. S., Li H. M., Xia J. X., Adv. Funct. Mater., 2020, 30(51), 2005834 |
| 70 | Wang J. Y., Cui Y., Wang D., Adv. Mater., 2019, 31(38), 1801993 |
| 71 | Zhao J. L., Yang M., Yang N. L., Wang J. Y., Wang D., Chem. Res. Chinese Universities, 2020, 36(3), 313—319 |
| 72 | He G., Evers S., Liang X., Cuisinier M., Garsuch A., Nazar L. F., ACS Nano, 2013, 7(12), 10920—10930 |
| 73 | Liang Z., Zheng G. Y., Li W. Y., Seh Z. W., Yao H. N., Yan K., Kong D. S., Cui Y., ACS Nano, 2014, 8(5), 5249—5256 |
| 74 | Mao D., Wan J. W., Wang J. Y., Wang D., Adv. Mater., 2019, 31(38), 1802874 |
| 75 | Ren H., Yu R. B., Wang J. Y., Jin Q., Yang M., Mao D., Kisailus D., Zhao H. J., Wang D., Nano Lett., 2014, 14(11), 6679—6684 |
| 76 | Wang C., Wang J. Y., Hu W. P., Wang D., Chem. Res. Chinese Universities, 2020, 36(1), 68—73 |
| 77 | Liu J. X., Tan Z. Q., Qu X. Z., Liang F. X., Yang Z. Z., Langmuir, 2022, 38(37), 11406—11413 |
| 78 | Zhao L. L., Zhu L. J., Chen Y., Wang Q., Li J. L., Zhang C. L., Liang F. X., Qu X. Z., Yang Z. Z., Chem. Commun., 2013, 49(55), 6161—6163 |
| 79 | Jang J. Y., Le T. M. D., Ko J. H., Ko Y. J., Lee S. M., Kim H. J., Jeong J. H., Thambi T., Lee D. S., Son S. U., Chem. Mater., 2019, 31(2), 300—304 |
| 80 | Seyed⁃Talebi S. M., Kazeminezhad I., Motamedi H., Ceram. Int., 2018, 44(12), 13457—13462 |
| 81 | Shao J. X., Abdelghani M., Shen G. Z., Cao S. P., Williams D. S., van Hest J. C. M., ACS Nano, 2018, 12(5), 4877—4885 |
| [1] | 宋佳欣, 崔静, 范晓强, 孔莲, 肖霞, 解则安, 赵震. 介孔二氧化硅负载高分散钒催化剂的制备及乙烷选择氧化性能[J]. 高等学校化学学报, 2023, 44(2): 20220532. |
| [2] | 王雅芝, 贾显枝, 张宏港, 刘璐, 赵彬然. 介质阻挡放电等离子制备5Ni-5La/SiO2催化剂及其甲烷干重整反应催化性能[J]. 高等学校化学学报, 2023, 44(2): 20220503. |
| [3] | 林俊旭, 习志威, 李志平, 王迎春. 钯催化炔丙醇与叔丁基异腈选择性合成吡咯并呋喃衍生物和氨基甲酸酯[J]. 高等学校化学学报, 2023, 44(2): 20220473. |
| [4] | 胡平澳, 张琪, 张会茹. 锂硫电池中硒缺陷WSe2催化性能的理论研究[J]. 高等学校化学学报, 2023, 44(2): 20220595. |
| [5] | 邓园, 王思, 丰海松, 张欣. Pd催化糠醛加氢反应中溶剂依赖效应的理论计算[J]. 高等学校化学学报, 2023, 44(2): 20220486. |
| [6] | 何建云, 蒋云波, 张爱敏, 唐振艳, 李鸿鹏. 新型卟啉基多孔有机聚合物COP-180负载钯催化剂的制备及应用[J]. 高等学校化学学报, 2023, 44(2): 20220535. |
| [7] | 刘双红, 夏思玉, 刘世奇, 李旻, 孙嘉杰, 钟永, 张锋, 白锋. 中空全固态Z型异质结光催化剂的研究进展[J]. 高等学校化学学报, 2023, 44(1): 20220512. |
| [8] | 王慧, 赵德偲, 杨乃亮, 王丹. 智能中空药物载体的门控设计[J]. 高等学校化学学报, 2023, 44(1): 20220237. |
| [9] | 李怀科, 岳贵初, 谢海韵, 刘静, 高松伟, 侯兰兰, 李帅, 苗贝贝, 王女, 白杰, 崔志民, 赵勇. 静电纺丝中空纳米纤维在催化领域的应用[J]. 高等学校化学学报, 2023, 44(1): 20220625. |
| [10] | 匡华艺, 陈晨. 贵金属纳米框架设计合成及电催化性能的研究进展[J]. 高等学校化学学报, 2023, 44(1): 20220586. |
| [11] | 杨庆凤, 吕良, 赖小勇. 中空MOFs材料制备及电催化应用的研究进展[J]. 高等学校化学学报, 2023, 44(1): 20220666. |
| [12] | 刘至辰, 张宏伟, 张博稳, 陈鹏, 袁珮. 吸附法制备金属/碳催化剂用于5-羟基甲基糠醛高效电催化氧化的研究[J]. 高等学校化学学报, 2023, 44(1): 20220631. |
| [13] | 李姿若, 张红娟, 朱国勋, 夏伟, 汤静. 负载酞菁铁的氮掺杂中空碳球的电催化氧还原性能[J]. 高等学校化学学报, 2023, 44(1): 20220677. |
| [14] | 唐全骏, 刘颖馨, 孟蓉炜, 张若天, 凌国维, 张辰. 单原子催化在海洋能源领域的应用[J]. 高等学校化学学报, 2022, 43(9): 20220324. |
| [15] | 楚宇逸, 兰畅, 罗二桂, 刘长鹏, 葛君杰, 邢巍. 单原子铈对弱芬顿效应活性位点氧还原稳定性的提升[J]. 高等学校化学学报, 2022, 43(9): 20220294. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||