

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (5): 1340.doi: 10.7503/cjcu20210001
收稿日期:2021-01-04
出版日期:2021-05-10
发布日期:2021-03-06
通讯作者:
于欣,刘宏
E-mail:ifc_yux@ujn.edu.cn;ifc_liuh@ujn.edu.cn
基金资助:
YANG Ruiqi, YU Xin( ), LIU Hong(
), LIU Hong( )
)
Received:2021-01-04
Online:2021-05-10
Published:2021-03-06
Contact:
YU Xin,LIU Hong
E-mail:ifc_yux@ujn.edu.cn;ifc_liuh@ujn.edu.cn
Supported by:摘要:
半导体光催化技术实现了太阳能向化学能的转化, 旨在解决日益严重的能源和环境问题, 达到可持续的能源利用. 由于大的比表面积和更多的表面缺陷, 纳米尺寸的催化剂表现出比块状材料更大的潜力. 目前, 四氧化三锡纳米材料因其生态友好和含量丰富而受到关注, 同时其具有合适的带隙(2.5~2.8 eV), 是一种极具潜力的新型可见光光催化剂. 本文综述了四氧化三锡基光催化纳米材料的最新研究进展, 从材料改性和应用两方面进行了阐述, 并展望了其未来发展方向, 为开发新型高效的四氧化三锡基纳米材料提供了指导.
中图分类号:
TrendMD:
杨瑞琪, 于欣, 刘宏. 四氧化三锡基光催化纳米材料的研究进展. 高等学校化学学报, 2021, 42(5): 1340.
YANG Ruiqi, YU Xin, LIU Hong. Scientific Study of Photocatalytic Material Based on Sn3O4. Chem. J. Chinese Universities, 2021, 42(5): 1340.
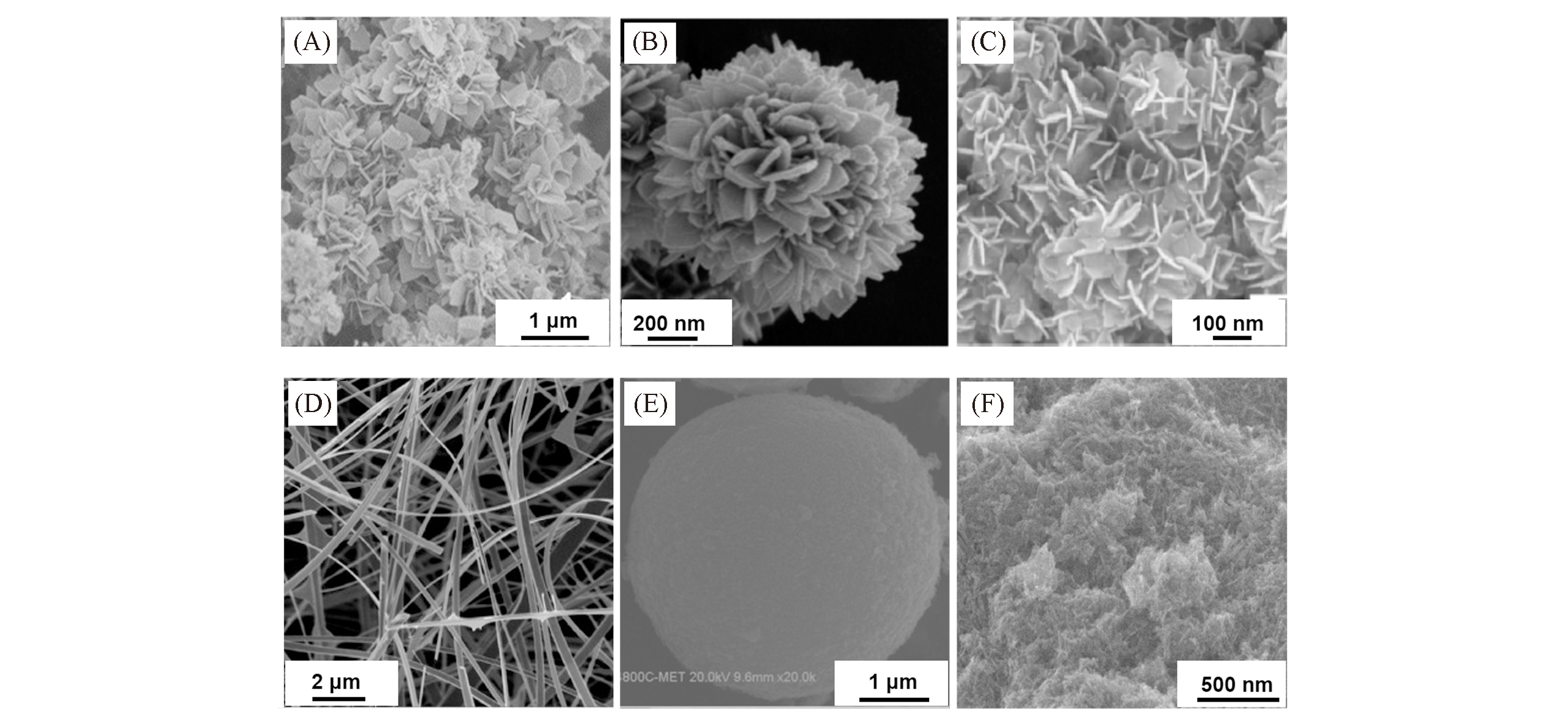
Fig.4 SEM images of Sn3O4 with different morphologies of nanoplates(A)[9], flower?like nanostructures(B)[10], nanosheets(C)[17], nanobelts(D)[21], microballs(E)[22] and nanowires(F)[23](A) Copyright 2014, American Chemical Society; (B) Copyright 2014, Royal Society of Chemistry; (C) Copyright 2015, Springer Nature; (D) Copyright 2010, Elsevier; (E) Copyright 2019, Elsevier; (F) Copyright 2020, Elsevier.
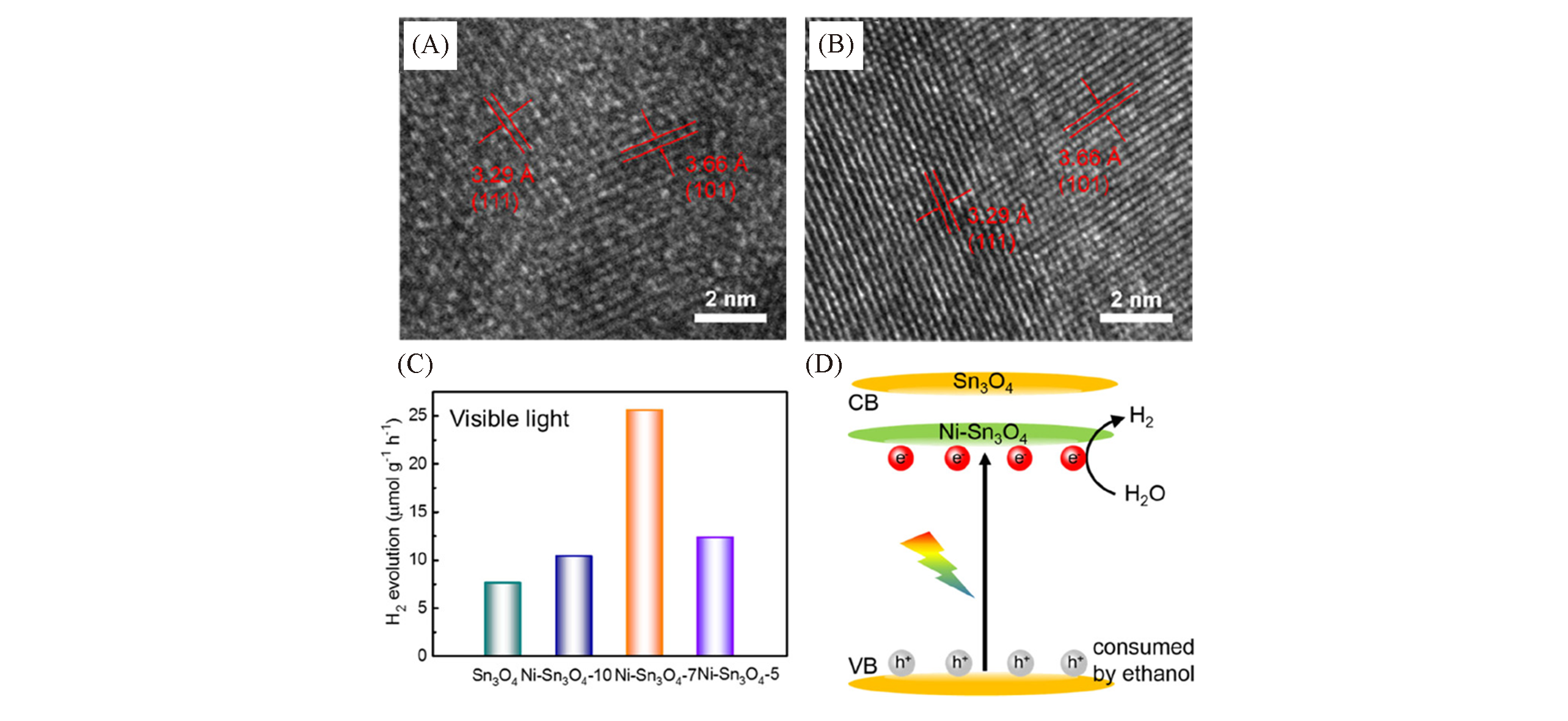
Fig.5 HRTEM images of Sn3O4(A) and Ni?Sn3O4(B), photocatalytic hydrogen production performance(C) and band alignment of Ni?Sn3O4(D)[24]Copyright 2020, American Chemical Society.
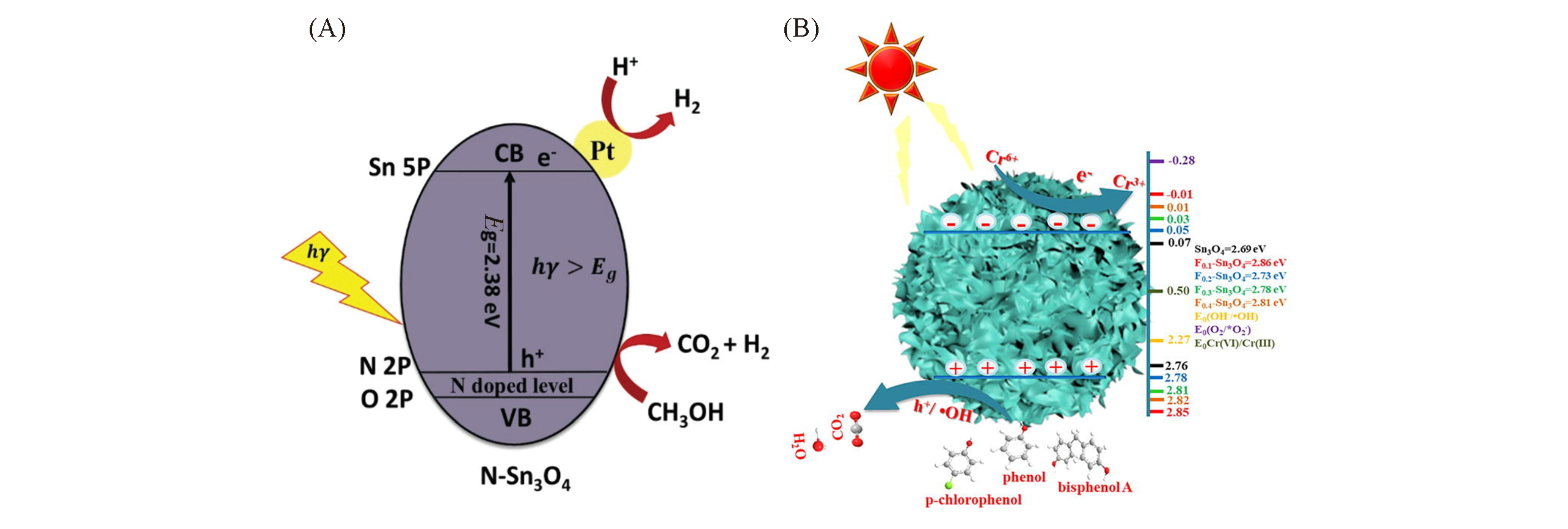
Fig.6 Photocatalytic mechanism of N?Sn3O4(A)[25] and F?Sn3O4 under light irradiation(B)[26](A) Copyright 2020, Royal Society of Chemistry; (B) Copyright 2020, Elsevier.
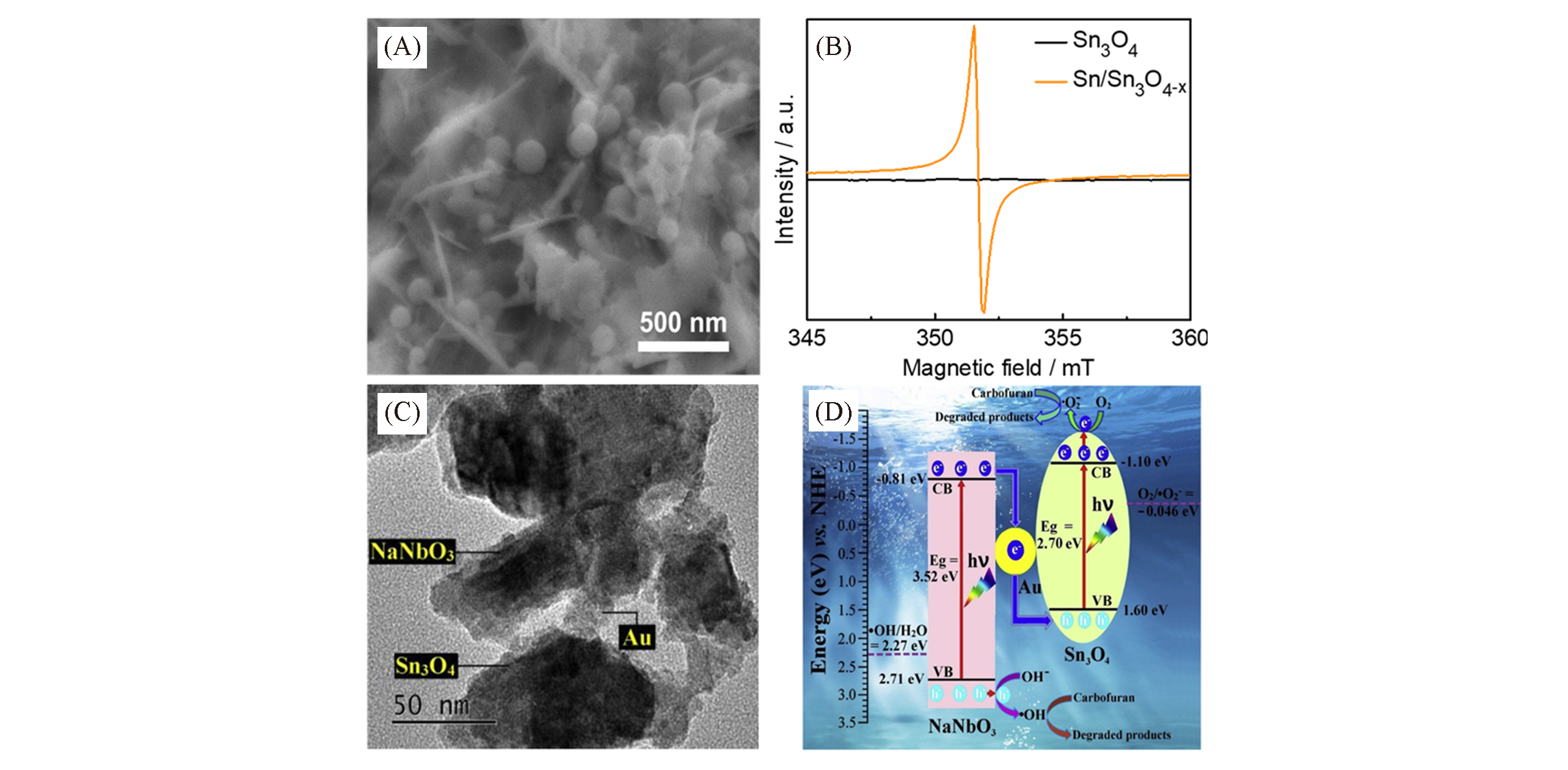
Fig.7 TEM image of Sn/Sn3O4-x(A) and EPR spectra of Sn3O4 and Sn/Sn3O4-xat room temperature(B)[30], TEM image NaNbO3?Au?Sn3O4(C) and possible photocatalytic degradation principle and process of NaNbO3?Au?Sn3O4 photocatalyst under sunlight irradiation(D)[31](A, B) Copyright 2020, Springer Nature; (C, D) Copyright 2020, Elsevier.
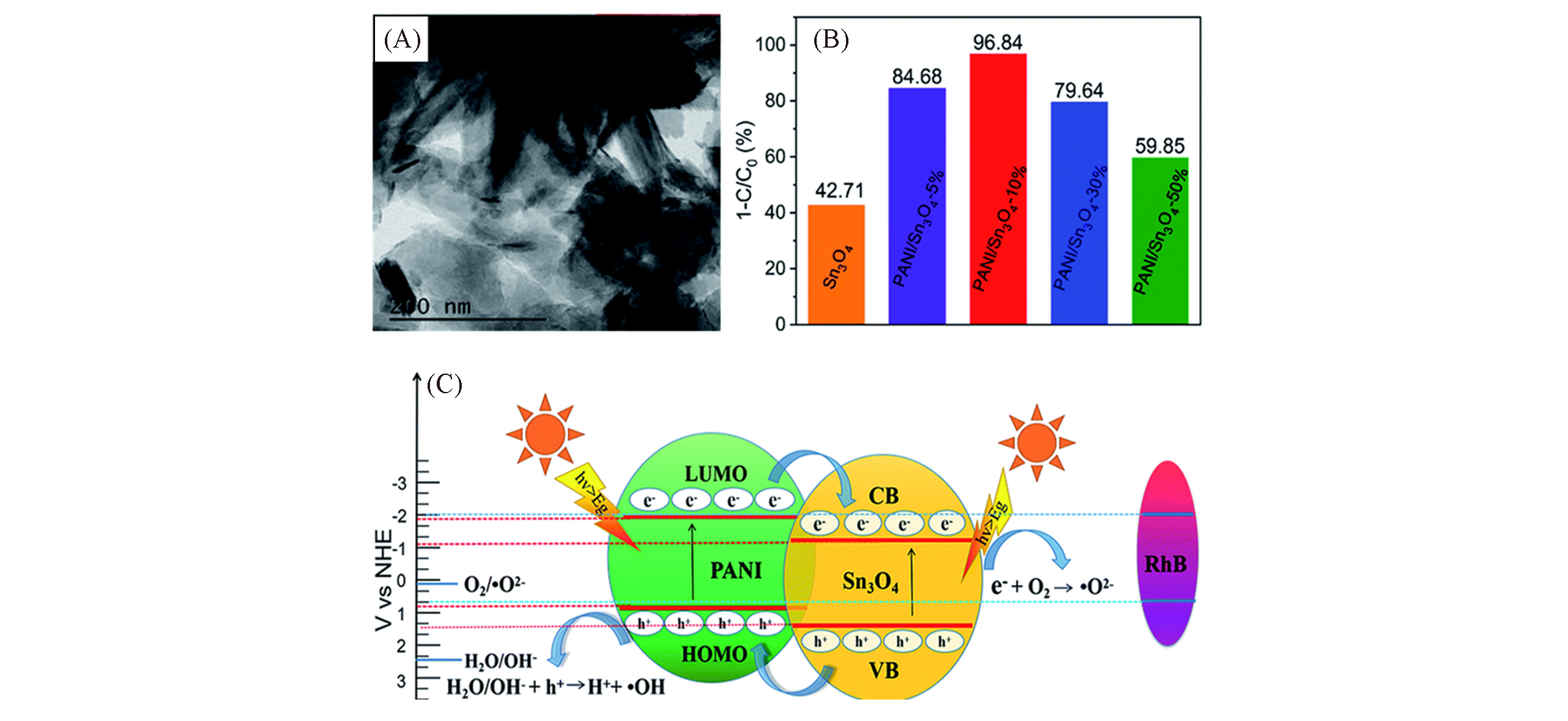
Fig.8 TEM image of PANI/Sn3O4 hybrid(A), photocatalytic performance of PANI/Sn3O4 composite with different PANI mass ratios on RhB dye degradation(B), schematic representation of the photocatalytic mechanism(C)[37]Copyright 2019, Royal Society of Chemistry.
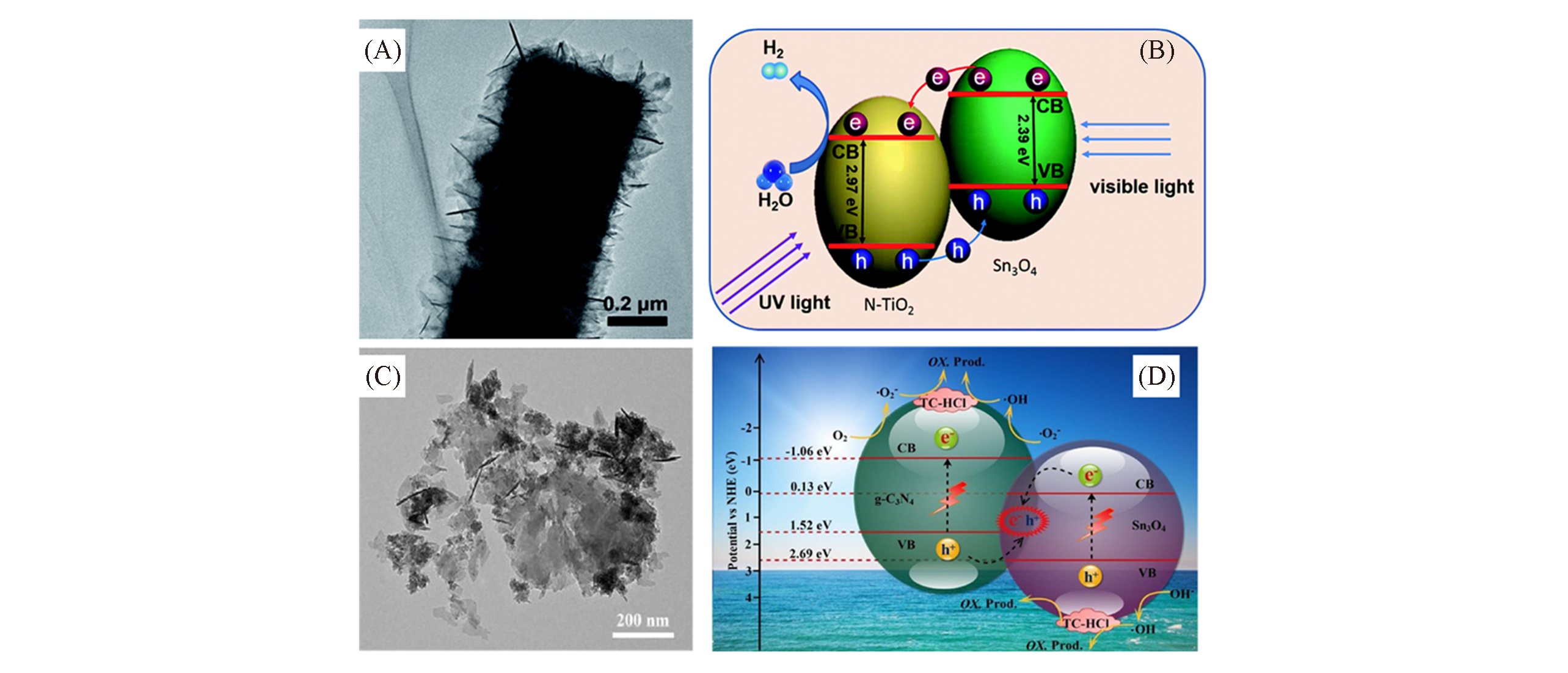
Fig.9 TEM images of Sn3O4 /N?TiO2(A)[39] and Sn3O4 /C3N4(C)[40], schematic diagram of Janus type Sn3O4 / N?TiO2(B)[39] and Z type Sn3O4 /C3N4 heterojunction photocatalytic reaction(D)[40](A, B) Copyright 2015, Royal Society of Chemistry; (C, D) Copyright 2018, Elsevier.
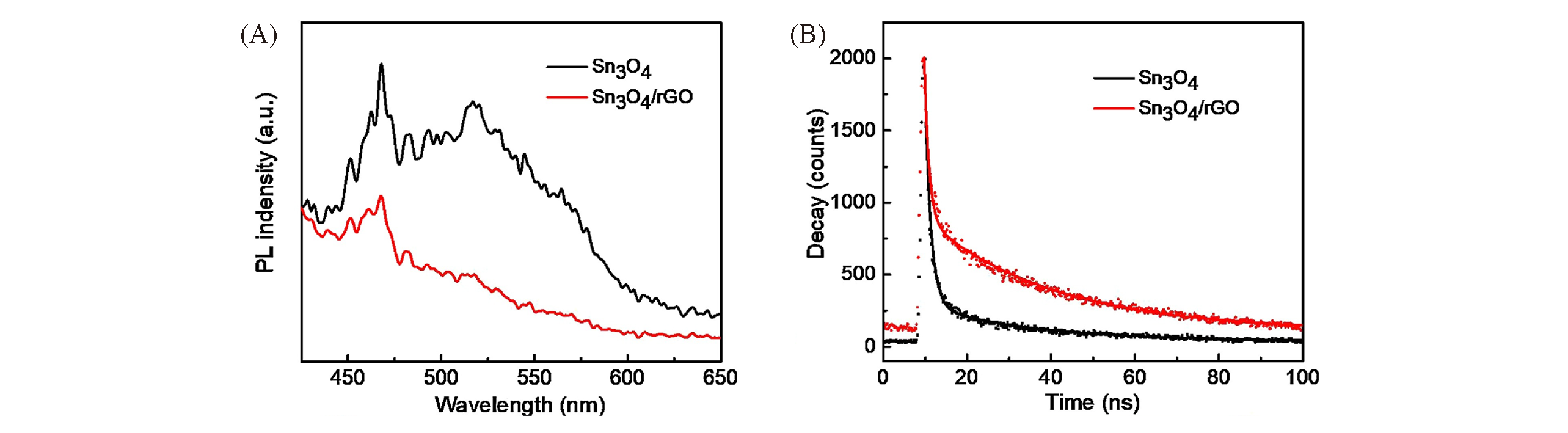
Fig.10 PL emission(A) and time?resolved PL decay spectra(B) of the Sn3O4 and Sn3O4/rGO at 450 nm exited by a 375 nm laser at room temperature[43]Copyright 2018, Elsevier.
| Sample | Decay time/ns | Relative amplitude(%) | Average lifetime/ns | ||
|---|---|---|---|---|---|
| τ1 | τ2 | f1 | f2 | τ* | |
| Sn3O4 | 1.41 | 26.12 | 23.85 | 76.15 | 25.71 |
| Sn3O4/rGO | 1.02 | 28.16 | 12.24 | 87.76 | 28.02 |
Table 1 Summary of the photoluminescence decay time(τ) and their relative amplitude(f) in the samples from the time-resolved PL spectra in Fig.10(B) by biexponential decays
| Sample | Decay time/ns | Relative amplitude(%) | Average lifetime/ns | ||
|---|---|---|---|---|---|
| τ1 | τ2 | f1 | f2 | τ* | |
| Sn3O4 | 1.41 | 26.12 | 23.85 | 76.15 | 25.71 |
| Sn3O4/rGO | 1.02 | 28.16 | 12.24 | 87.76 | 28.02 |
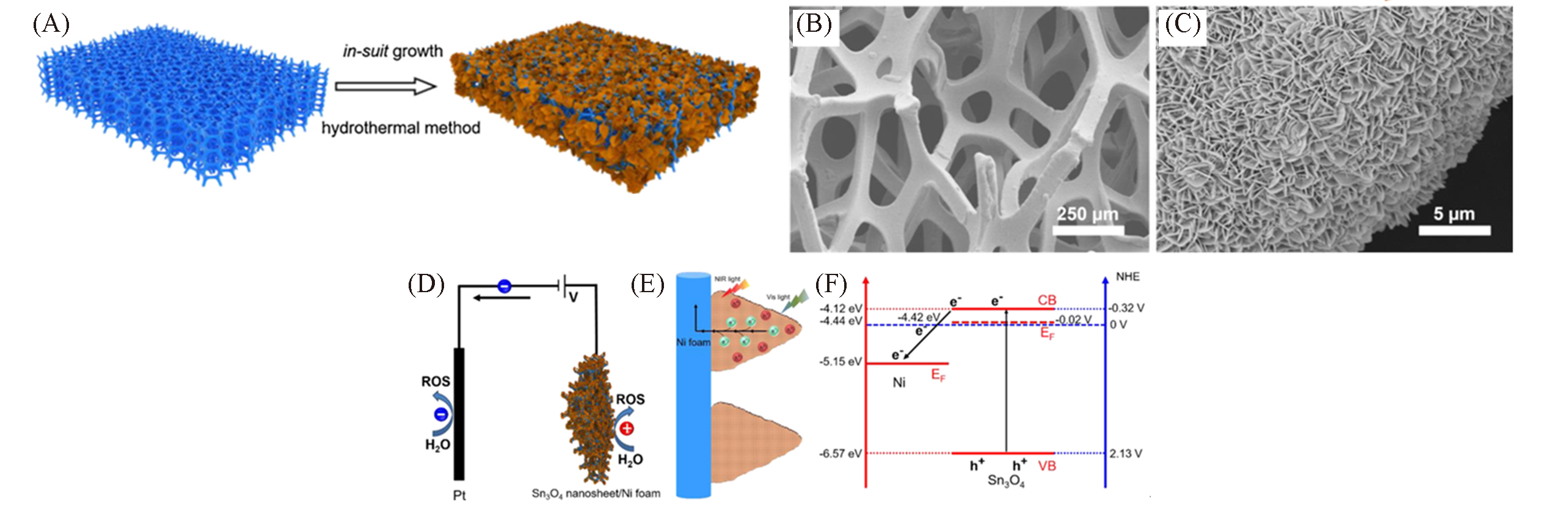
Fig.11 Graphical representation of the preparation of the Sn3O4 nanosheet/Ni foam heterostructure(A), SEM images of Sn3O4 nanosheet/Ni foam heterostructure(B, C), schematic diagram of photoelectrocatalysis(D), schematic diagram of photogenerated carrier separation(E), band alignment of the Sn3O4 nanosheet/Ni foam heterostructure(EF: the Fermi level)(F)[46]Copyright 2019, Elsevier.
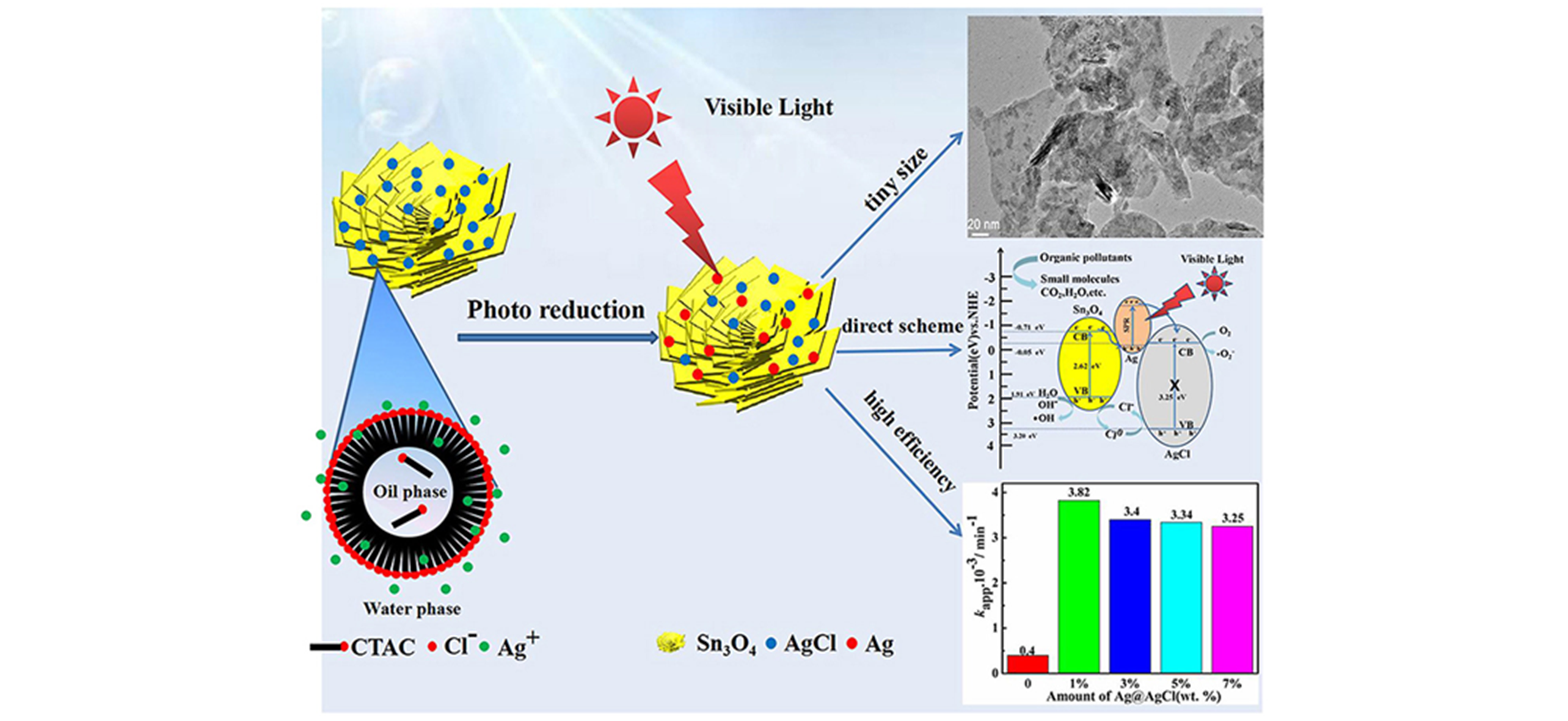
Fig.12 Schematic illustration of preparation, characterization of morphology and photocatalytic performance and schematic diagram of photocatalytic mechanism of Ag@AgCl/Sn3O4 photocatalysts[47]Copyright 2020, Elsevier.
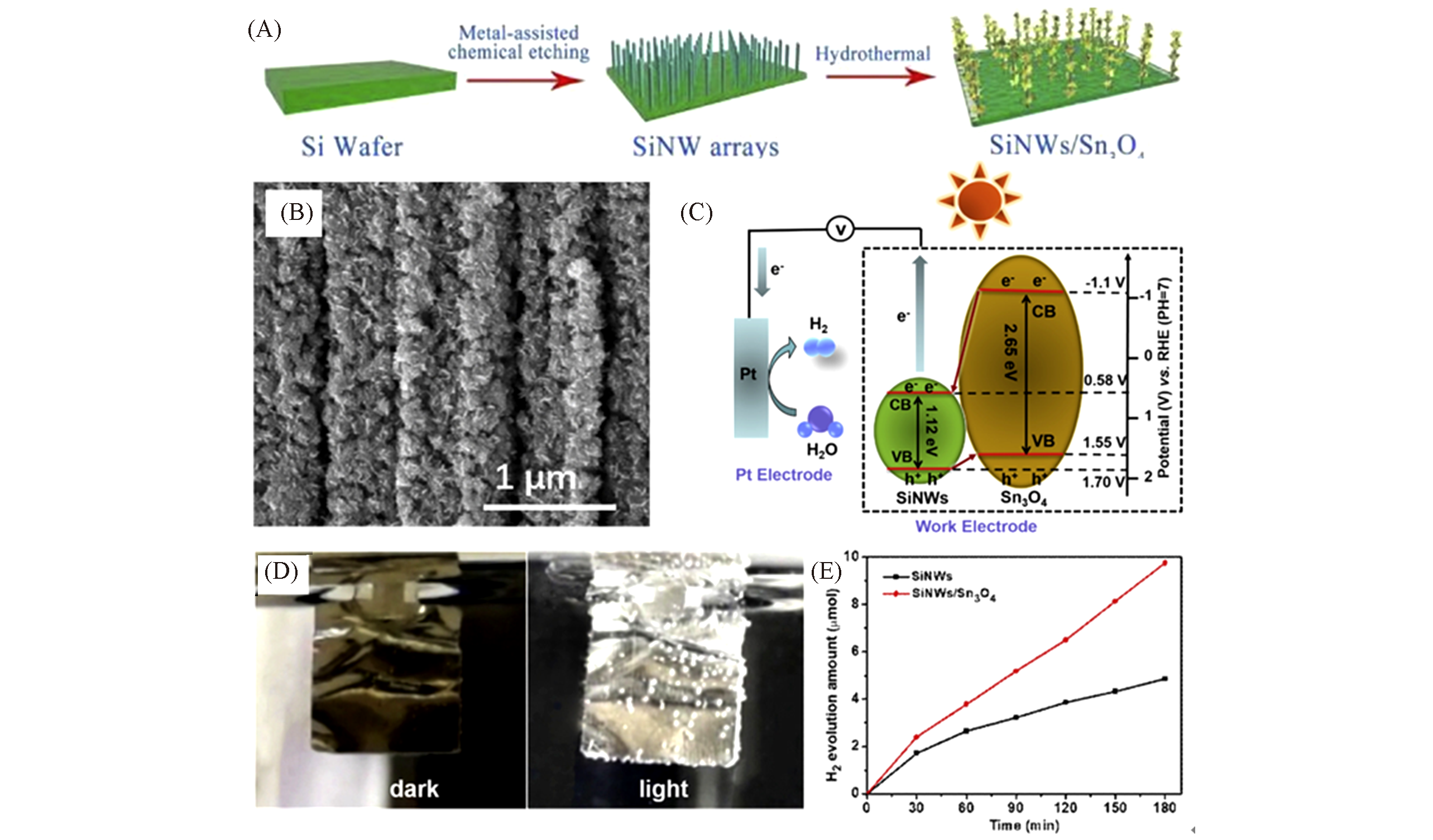
Fig.13 Schematic diagram of the formation for SiNWs/Sn3O4 nanocomposites(A), SEM image of SiNWs/Sn3O4 nanocomposites(B), schematic diagram of electron transfer in the SiNWs/Sn3O4(C), the photographs of H2 generation on Pt counter electrode(D) and photoelectrocatalytic H2 generation of SiNWs and SiNWs/Sn3O4 photoanode(E)[49](D) Off light condition(left) and on light condition(right); SiNWs/Sn3O4 hierarchical heterostructured array is used as working electrode for photoelectrocatalytic water splitting. Copyright 2019, Elsevier.
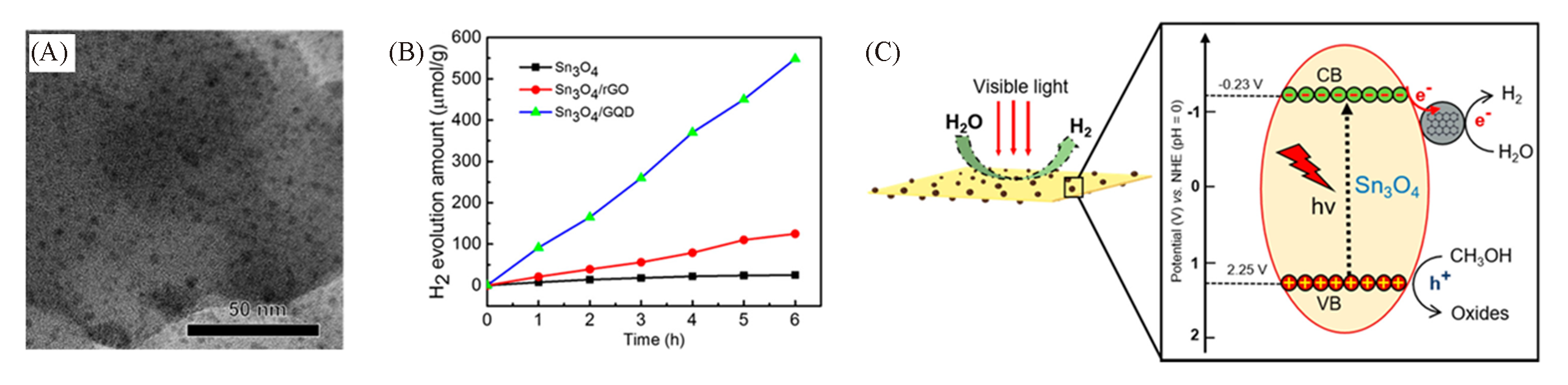
Fig.14 TEM image of Sn3O4 /GQD(A), photocatalytic activity assessment: photocatalytic H2 evolution from Sn3O4, Sn3O4 /rGO and Sn3O4 /GQD(B), schematic model for the photocatalytic process of the Sn3O4 /GQD planar nano?heterojunction under visible light(C)[52]Copyright 2018, American Chemical Society.
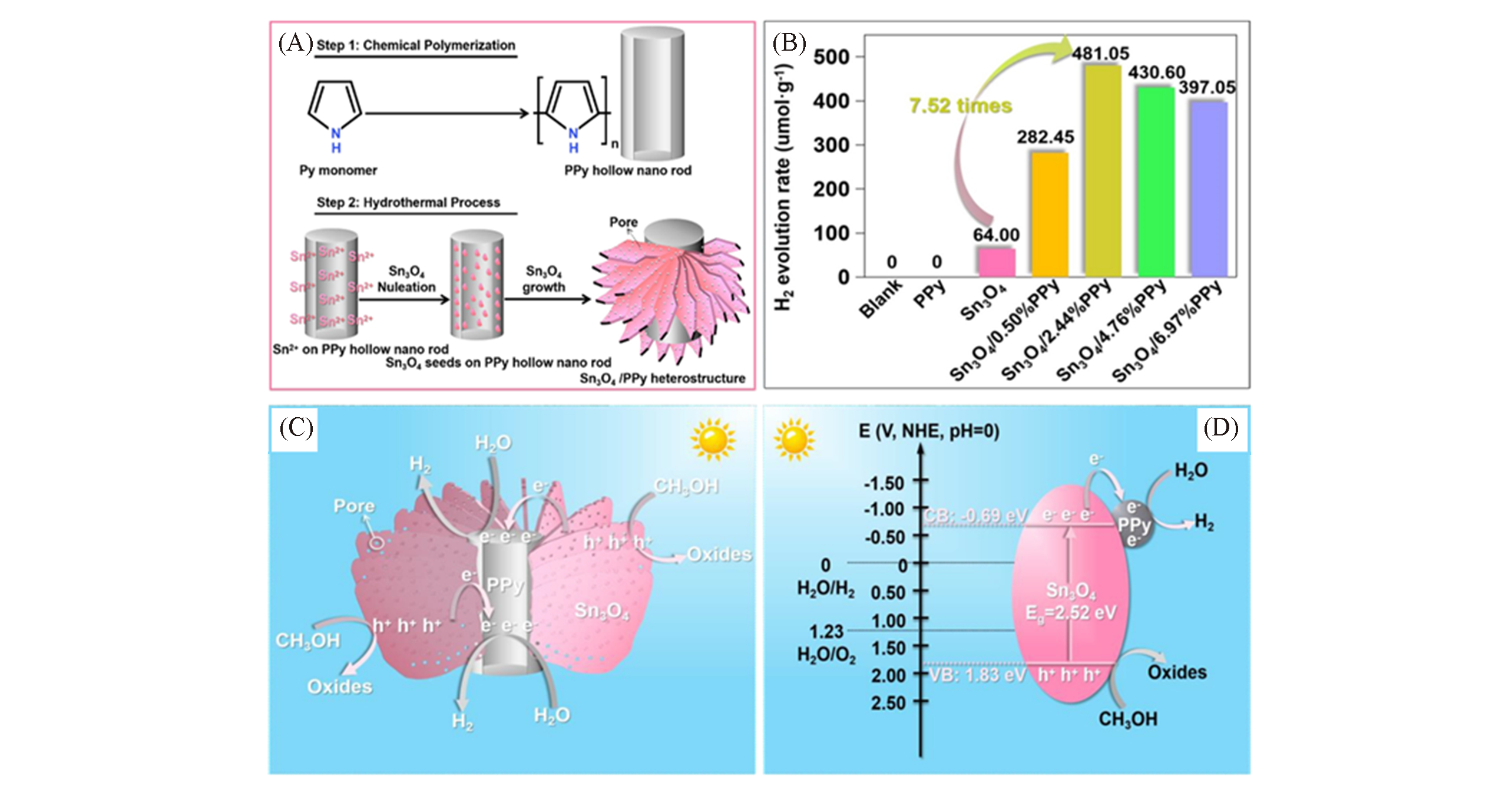
Fig.15 Schematic representation of the synthetic procedure leading to Sn3O4 /PPy heterostructure(A), H2 photo?evolution amount within 5 h(B), schematic diagram of the plausible mechanism for H2 photo?evolution reaction catalyzed by Sn3O4 /PPy composite(C), schematic representation of energy?level diagram showing electron transfer paths from Sn3O4 to PPy(D)[55]Copyright 2020, Elsevier.
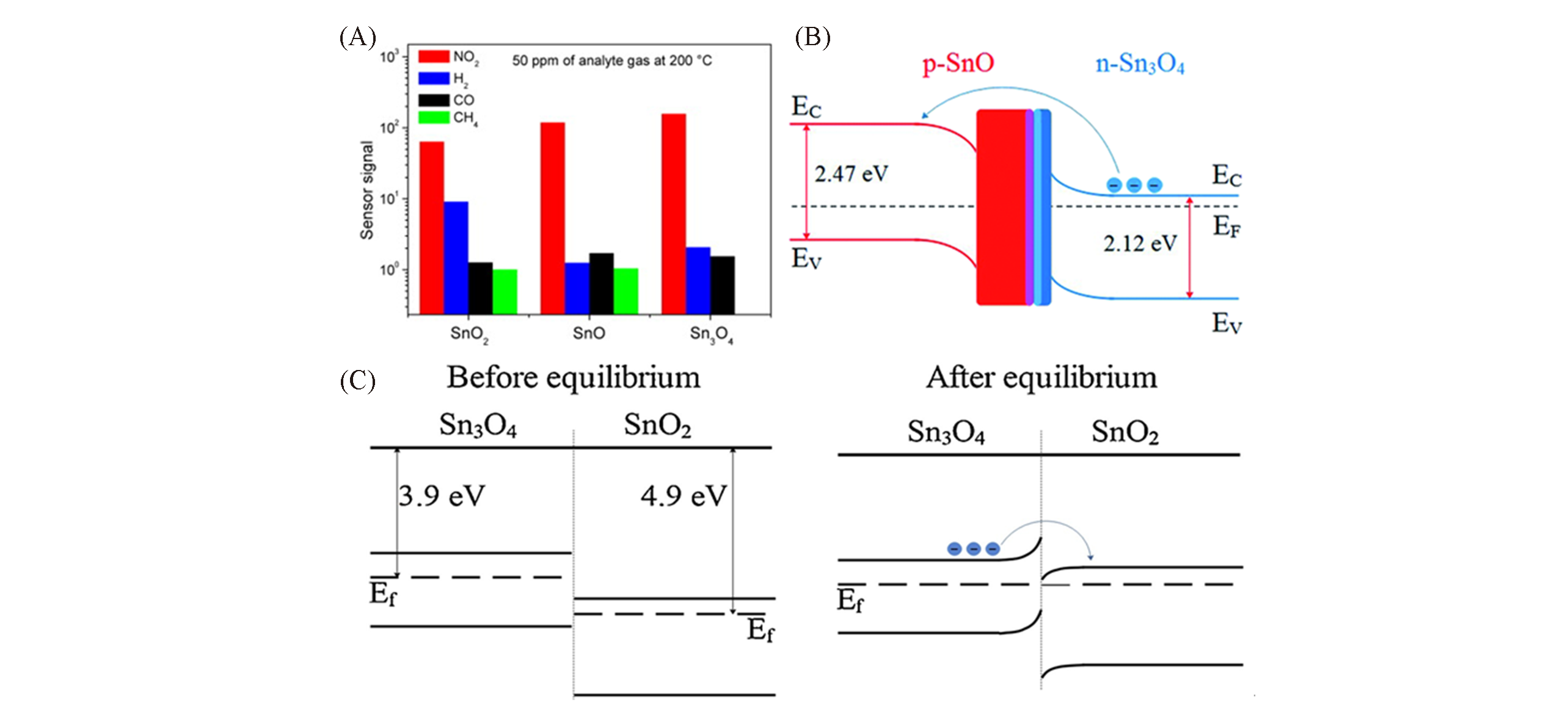
Fig.16 Sensor signal of the three tin oxide nanobelts compositions for 6.4×10-5 g/L of NO2, H2, CO and CH4 at 200 ℃(A)[63], schematic energy band diagram of SnO?Sn3O4 heterostructure(B)[64], schematic energy band diagram of SnO2?Sn3O4 heterostructure(C)[65](A) Copyright 2015, Elsevier; (B) Copyright 2020, Royal Society of Chemistry; (C) Copyright 2019, Elsevier.
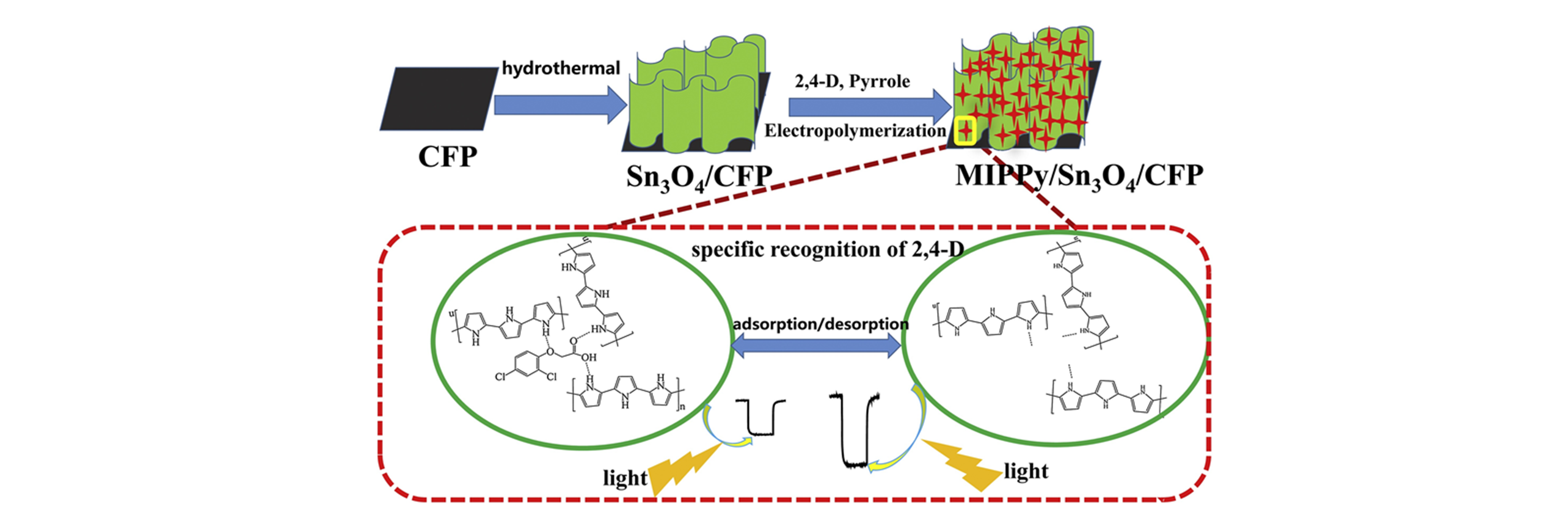
Fig.17 Schematic illustration of the synthesis procedure of Sn3O4 nanoflakes and MIPPy/Sn3O4@CFP and their application for 2,4?D detection[68]Copyright 2018, Elsevier.
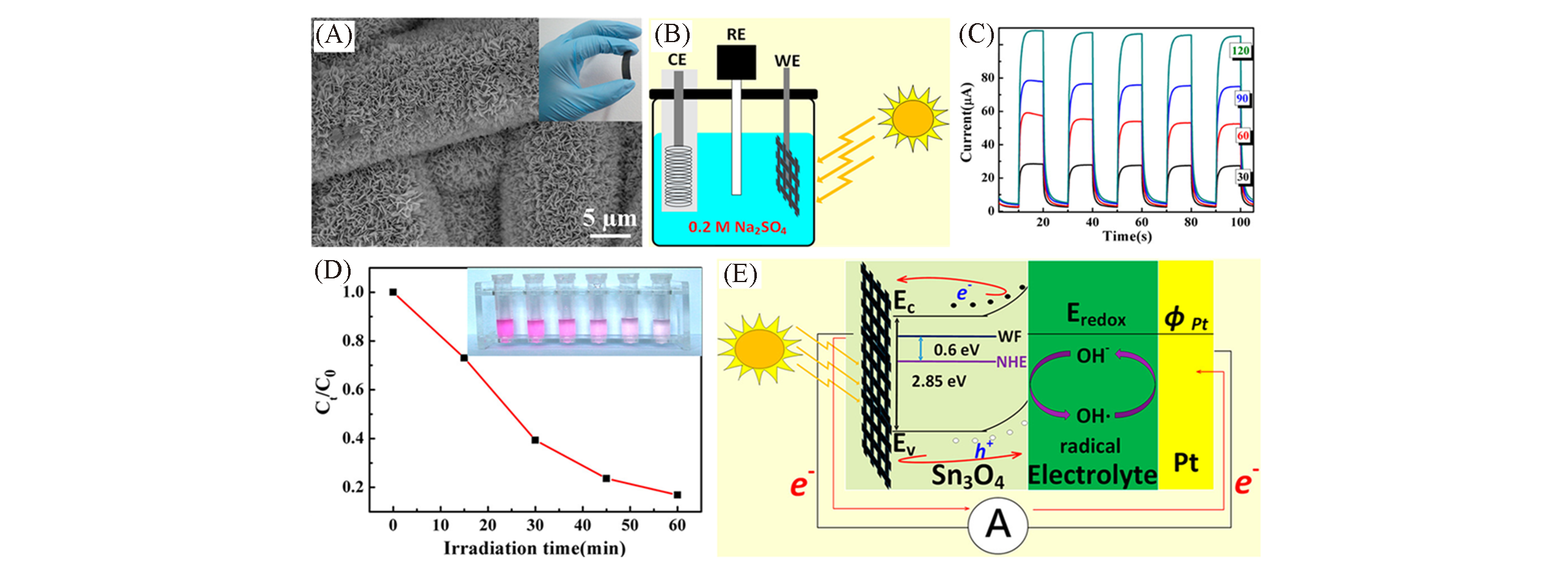
Fig.18 SEM image of Sn3O4/carbon fiber paper(A), schematic illustration of a self?powered PEC?type detector(B), photocurrent response under on/off cycling at 0 V(vs. Ag/AgCl) for incident intensity equal to 30, 60, 90, and 120 mW/cm2(C), degradation efficiency with irradiation time under visible?light irradiation and photograph of the color change of RhB during the photodegradation process(inset)(D), positions of the band gap for Sn3O4(E)[70]Copyright 2017, American Chemical Society.
| 33 | Wang H., Liu W., Ma J., Liang Q., Qin W., Lartey O. P., Feng X., Int. J. Miner. Metall. Mater., 2020, 27(6), 830—839 |
| 34 | Yu X., Zhang J., Zhao Z. H., Guo W. B., Qiu J. C., Mou X. N., Li A. X., Claveria J. P., Liu H., Nano Energy, 2015, 16, 207—217 |
| 35 | Akti F., Appl. Surf. Sci., 2018, 455, 931—939 |
| 36 | Lin Y., Wu S., Yang C., Chen M., Li X., Appl. Catal. B: Environ., 2019, 245, 71—86 |
| 37 | Lv M. F., Yang L. Q., Wang X. L., Cheng X. L., Song Y., Yin Y. K., Liu H. M., Han Y. J., Cao K. S., Ma W., Qi G., Li S. T., RSC Adv., 2019, 9(69), 40694—40707 |
| 38 | Ji Y., Song G., Yang R., Ding L., Wang A., Ren N., Zhang J., Yu X., J. Nanosci. Nanotechnol., 2021, 21, 2647—2652 |
| 39 | Yu X., Wang L. F., Zhang J., Guo W. B., Zhao Z. H., Qin Y., Mou X. N., Li A. Z., Liu H., J. Mater. Chem. A, 2015, 3(37), 19129—19136 |
| 40 | Li C. M., Yu S. Y., Dong H. J., Liu C. B., Wu H. J., Che H. N., Chen G., Appl. Catal. B: Environ., 2018, 238, 284—293 |
| 41 | Hu J. L., Li X. Y., Wang X. D., Li Q. S., Wang F. P., Dalton Trans., 2019, 48(24), 8937—8947 |
| 42 | Sun M., Yan T., Wu T. T., He Y. H., Shao Y., Wei D., Du B., Mater. Res. Bull., 2018, 103, 104—114 |
| 43 | Yu X., Zhao Z. H., Sun D. H., Ren N., Yu J. H., Yang R. Q., Liu H., Appl. Catal. B: Environ., 2018, 227, 470—476 |
| 44 | Yu X., Ren N., Qiu J., Sun D., Li L., Liu H., Sol. Energy Mat. Sol. Cells, 2018, 183, 41—47 |
| 45 | Xiao J. D., Xie Y. B., Li C. H., Kim J. H., Tang K. X., Cao H. B., Catal. Today, 2018, 307, 147—153 |
| 46 | Yang R. Q., Ji Y. C., Zhang J., Zhang R. T., Liu F., Chen Y. K., Liang L. L., Han S. W., Yu X., Liu H., Catal. Today, 2019, 335, 520—526 |
| 47 | Han Y. Q., Wei M. M., Qu S. Y., Zhong M., Han L. J., Yang H. D., Liu Y., Su B. T., Lei Z. Q., Ceram. Int., 2020, 46, 24060—24070 |
| 48 | Yu X., Zhao Z., Zhang J., Guo W., Li L., Liu H., Wang Z. L., CrystEngComm, 2017, 19, 129—136 |
| 49 | Yang R. Q., Ji Y. C., Li Q, Zhao Z. H., Zhang R. T., Liang L. L., Liu F., Chen Y. K., Han S. W., Yu X., Liu H., Appl. Catal. B: Environ., 2019, 256, 117798 |
| 50 | Wang L., Gao F., Wang A., Chen X., Li H., Zhang X., Zheng H., Ji R., Li B., Yu X., Liu J., Gu Z., Chen F., Chen C., Adv. Mater., 2020, 32(48), 2005423 |
| 51 | Zhang Z., Zhang J., Chen N., Qu L., Energy Environ. Sci., 2012, 5(10), 8869—8890 |
| 52 | Yu X., Zhao Z. H., Ren N., Liu J., Sun D. H., Ding L. H., Liu H., ACS Sustain. Chem. Eng., 2018, 6(9), 11775—11782 |
| 53 | Carraher C. E., Battin A. J., Roner M. R., J. Inorg. Organomet. Polym. Mater., 2013, 23(1), 61—73 |
| 54 | Li W., Zhang Q., Zheng G., Seh Z. W., Yao H., Cui Y., Nano Lett., 2013, 13(11), 5534—5540 |
| 55 | Yang L. Q., Lv M. F., Song Y., Yin K. Y., Wang X. L., Cheng X. L., Cao K. S., Li S. T., Wang C., Yao Y. F., Luo W. J., Zou Z. G., Appl. Catal. B: Environ., 2020, 279, 119341 |
| 56 | Xu L., Chen W. Q., Ke S. Q., Zhang S. M., Zhu M., Zhang Y., Shi W. Y., Horike S. S., Tang L., Chem. Eng. J., 2020, 382, 122810 |
| 57 | Halmann M., Nature, 1978, 275(5676), 115—116 |
| 58 | Francke R., Schille B., Roemelt M., Chem. Rev., 2018, 118(9), 4631—4701 |
| 59 | Chen Z., Gao M. R., Duan N. Q., Zhang J. G., Zhang Y. Q., Fan T. T., Zhang J. W., Dong Y. Y., Li J. H., Liu Q. X., Yi X. D., Luo J. L., Appl. Catal. B: Environ., 2020, 277, 119252 |
| 60 | Han N., Ding P., He L., Li Y., Li Y., Adv. Energy Mater., 2019, 10, 1902338 |
| 61 | Liu L. X., Zhou Y., Chang Y. C., Zhang J. R., Jiang L. P., Zhu W. L., Lin Y. H., Nano Energy, 2020, 77, 105296 |
| 62 | Kim I. D., Rothschild A., Tuller H. L., Acta Mater., 2013, 61(3), 974—1000 |
| 63 | Sumana P. H., Felixa A. A., Tuller H. L., Varela J. A., Orlandi M. O., Sens. Actuators B: Chem., 2015, 208, 122—127 |
| 64 | Zeng W. W., Liu Y. Z., Chen G. L., Zhan H. R., Mei J., Luo N., He Z. K., Tang C. Y., RSC Adv., 2020, 10(50), 29843—29854 |
| 65 | Zeng W. W., Liu Y. Z., Mei J., Tang C. Y., Luo K., Li S. M., Zhan H. R., He Z. K., Sens. Actuators B: Chem., 2019, 301, 127010 |
| 66 | Yu X., Zhao Z., Zhang J., Guo W., Qiu J., Li D., Li Z., Mou X., Li L., Li A., Liu H., Small, 2016, 12(20), 2759—2767 |
| 67 | Wu W., Bai S., Yuan M., Qin Y., Wang Z. L., Jing T., ACS Nano, 2012, 6(7), 6231—6235 |
| 68 | Wang J., Xu Q., Xia W. W., Shu Y., Jin D. Q., Zang Y., Hu X. Y., Sens. Actuators B: Chem., 2018, 271, 215—224 |
| 69 | Wang H. M., Xu Q., Wang J., Du W., Liu F. P., Hu X. Y., Biosens. Bioelectro., 2018, 100, 105—114 |
| 70 | Xia W., Qian H., Zeng X., Dong J., Wang J., Xu Q., J. Phy. Chem. C, 2017, 121, 19036—19043 |
| 71 | Xu R., Du Y., Leng D. Q., Liu L., Li Y. Y., Ren X., Fan D. W., Wang H., Wei Q., Chem. Commun., 2020, 56, 7455—7458 |
| 1 | Fujishima A., Honda K., Nature, 1972, 238(5358), 37—38 |
| 2 | Carey J. H., Lawrence J., Tosine H. M., Bull. Environ. Contam. Toxicol., 1976, 16(6), 697—701 |
| 3 | Sun Z., Talreja N., Tao H., Texter J., Muhler M., Strunk J., Chen J., Angew. Chem. Int. Ed., 2017, 57(26), 7610—7627 |
| 4 | Wang L., Zhang X., Yu X., Gao F., Shen Z., Zhang X., Ge S., Liu J., Gu Z., Chen C., Adv. Mater., 2019, 31(33), 1901965 |
| 5 | Yu X., Jin X., Chen X., Wang A., Zhang J., Zhang J., Zhao Z., Gao M., Razzari L., Liu H., ACS Nano, 2020, 14(10), 13876—13885 |
| 6 | Ji Y., Yang R., Wang L., Song G., Wang A., Lv Y., Gao M., Zhang J., Yu X., Chem. Eng. J., 2020, 402, 1262226 |
| 7 | Yu X., Zhao Z. Z., Sun D. H., Ren N., Ding L. H., Yang R. Q., Ji Y. C., Li L. L., Liu H, Chem. Commun., 2018, 54, 6056—6059 |
| 8 | Zheng J. Y., Lyu Y. H., Xie C., Wang R. L., Tao L., Wu H. B., Zhou H. J., Jiang S. P., Wang S. Y., Adv. Mater., 2018, 30(31), 1801773 |
| 9 | Manikandan M., Tanabe T., Li P., Ueda S., Ramesh G. V., Kodiyath R., Wang J. J., Hara T., Dakshanamoorthy A., Ishihara S., Ariga K., Ye J. H., Umezawa N., Abe H., ACS Appl. Mater. Interfaces, 2014, 6(6), 3790—3793 |
| 10 | He Y. H., Li D. Z., Chen J., Shao Y., Xian J. J., Zheng X. Z., Wang P., RSC Adv., 2014, 4(3), 1266—1269 |
| 11 | Berengue O. M., Simon R. A., Chiquito A. J., Dalmaschio C. J., Leite E. R., Guerreiro H. A., Guimarães F. E. G., J. Appl. Phys., 2010, 107(3), 033717 |
| 12 | Villars P., O⁃Sn Binary Phase Diagram 30—100 at.% Sn, Material Phases Data System(MPDS), SpringerMaterials, Uitznau, sd_1703036, sd_0304865, 2014 |
| 13 | Wang J. J., Umezawa N., Hosono H., Adv. Energy Mater., 2016, 6(1), 1501190 |
| 14 | Tanabe T., Tanikawa T., Nakamori K., Ueda S., Nanzai B., Matsubara Y., Matsumoto F., Int. J. Hydrog. Energy, 2020, 45(53), 28607—28615 |
| 15 | Balgude S. D., Sethi Y. A., Kale B. B., Munirathnam N. R., Amalnerkarb D. P., Adhyapak P. V., RSC Adv., 2016, 6(98), 95663—95669 |
| 16 | Deng H. M., Hossenlopp J. M., J. Phys. Chem. B, 2005, 109(1), 66—73 |
| 17 | Song H., Son S. Y., Kim S. K., Gun Y. J., Nano Res., 2015, 8(11), 3553—3561 |
| 18 | Song G., Wang L., Yang R., Ji Y., Zhang R., Yang L., Ding L., Wang A., Ren N., Yu X., J. Nanosci. Nanotechnol., 2020, 20, 5943—5949 |
| 19 | Liu Z., Wang L., Yu X., Zhang J., Yang R., Zhang X., Ji Y., Wu M., Deng L., Li L., Wang Z. L., Adv. Funct. Mater., 2019, 29, 1807279 |
| 20 | Xia W. W., Wang H. B., Zeng X. H., Han J., Zhu J., Zhou M., Wu S. D., CrystEngComm, 2014, 16(30), 6841—6847 |
| 21 | Damaschio C. J., Berengue O. M., Stroppa D. G., Simon R. A., Ramirez A. J., Schreiner W. H., Chiquito A. J., Leite E. R., J. Cryst. Growth, 2010, 312(20), 2881—2886 |
| 22 | Balgude S., Sethi Y., Kale B., Amalnerkar D., Adhyapak P., Mater. Chem. Phys., 2019, 221, 493—500 |
| 23 | Mone P., Mardikar S., Balgude S., Nano⁃Structures and Nano⁃bjects, 2020, 24, 100615 |
| 24 | Yang R. Q., Ji Y. C., Wang L. W., Song G. X., Wang A. Z., Ding L. H., Ren N., Lv Y. W., Zhang J., Yu X., ACS Appl. Nano Mater., 2020, 3(9), 9268—9275 |
| 25 | Balgude S., Sethi Y., Gaikwad A., Kale B., Amalnerkard D., Adhyapak P., Nanoscale, 2020, 12, 8502—8510 |
| 26 | Zeng D. B., Yu C. L., Fan Q. Z., Zeng J. L., Wei L. F., Li Z. S., Yang K., Ji H. B., Chem. Eng. J., 2020, 391, 123607 |
| 27 | Zhou Z. K., Peng X. N., Yang Z. J., Zhang Z. W., Li M., Su X. R., Zhang Q., Shan X. Y., Wang Q. Q., Zhang Z. Y., Nano Lett., 2011, 11(1), 49—55 |
| 28 | Li M. Y., Yuan N., Tang Y. W., Pei L., Zhu Y. D., Liu J. X., Bai L. H., Li M. Y., J. Mater. Sci. Technol., 2019, 35(4), 604—609 |
| 29 | Cheng Z., Ma Y., Yang L., Cheng F., Huang Z. J., Natan A., Li H. Y., Chen Y., Cao D. X., Huang Z. F., Wang Y. H., Liu Y. M., Yang R. D., Zhu H. L., Adv. Opt. Mater., 2019, 7(9), 1801816 |
| 30 | Yang R. Q., Liang N., Chen X. Y., Wang L. W., Song G. X., Ji Y. C., Ren N., Lv Y. W., Zhang J., Yu X., Int. J. Miner. Metall. Mater., 2021, 18, 150—159 |
| 31 | Li S. G., Liu Z. Y., Qu Z. H., Piao C. C., Liu J. Z., Xu D., Li X. J., Wang J., Song Y. T., J. Photochem. Photobiol. A, 2020, 389, 112246 |
| 32 | Yu X., Wang S., Zhang X., Qi A., Qiao X., Liu Z., Wu M., Li L., Wang Z. L., Nano Energy, 2018, 46, 29—38 |
| [1] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [2] | 江博文, 陈敬轩, 成永华, 桑微, 寇宗魁. 单原子材料在电化学生物传感中的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220334. |
| [3] | 林治, 彭志明, 贺韦清, 沈少华. 单原子与团簇光催化: 竞争与协同[J]. 高等学校化学学报, 2022, 43(9): 20220312. |
| [4] | 滕镇远, 张启涛, 苏陈良. 聚合物单原子光催化剂的载流子分离和表面反应机制[J]. 高等学校化学学报, 2022, 43(9): 20220325. |
| [5] | 李玉龙, 谢发婷, 管燕, 刘嘉丽, 张贵群, 姚超, 杨通, 杨云慧, 胡蓉. 基于银离子与DNA相互作用的比率型电化学传感器用于银离子的检测[J]. 高等学校化学学报, 2022, 43(8): 20220202. |
| [6] | 郭程, 张威, 唐云. 有序介孔材料: 历史、 现状与发展趋势[J]. 高等学校化学学报, 2022, 43(8): 20220167. |
| [7] | 夏雾, 任颖异, 刘京, 王锋. 壳聚糖包裹CdSe量子点组装体的水相可见光催化CO2还原[J]. 高等学校化学学报, 2022, 43(7): 20220192. |
| [8] | 赵盈喆, 张建玲. 金属-有机框架基材料在二氧化碳光催化转化中的应用[J]. 高等学校化学学报, 2022, 43(7): 20220223. |
| [9] | 邱丽琪, 姚向阳, 何良年. 可见光驱动丰产金属卟啉类配合物催化的二氧化碳选择性还原反应[J]. 高等学校化学学报, 2022, 43(7): 20220064. |
| [10] | 龚妍熹, 王建兵, 柴歩瑜, 韩元春, 马云飞, 贾超敏. 钾掺杂g-C3N4薄膜光阳极的制备及光电催化氧化降解水中双氯芬酸钠性能[J]. 高等学校化学学报, 2022, 43(6): 20220005. |
| [11] | 王广琦, 毕艺洋, 王嘉博, 石洪飞, 刘群, 张钰. 非贵金属三元复合Ni(PO3)2-Ni2P/CdS NPs异质结的构建及可见光高效催化产氢性能[J]. 高等学校化学学报, 2022, 43(6): 20220050. |
| [12] | 宋颖颖, 黄琳, 李庆森, 陈立妙. CuO/BiVO4光催化剂的制备及光催化CO2还原性能[J]. 高等学校化学学报, 2022, 43(6): 20220126. |
| [13] | 王君旸, 刘争, 张茜, 孙春燕, 李红霞. DNA银纳米簇在功能核酸荧光生物传感器中的应用[J]. 高等学校化学学报, 2022, 43(6): 20220010. |
| [14] | 陶雨, 欧鸿辉, 雷永鹏, 熊禹. 单原子催化剂在光催化二氧化碳还原中的研究进展[J]. 高等学校化学学报, 2022, 43(5): 20220143. |
| [15] | 冯丽, 邵兰兴, 李思骏, 全文选, 庄金亮. 超薄Sm-MOF纳米片的合成及可见光催化降解芥子气模拟剂性能[J]. 高等学校化学学报, 2022, 43(4): 20210867. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||