

高等学校化学学报 ›› 2018, Vol. 39 ›› Issue (12): 2611.doi: 10.7503/cjcu20180009
收稿日期:2018-01-03
出版日期:2018-09-10
发布日期:2018-09-10
作者简介:联系人简介: 李 夏, 女, 博士, 教授, 博士生导师, 主要从事无机配位化学研究. E-mail:
基金资助:
ZHANG Dechun1,2, XU Qiwei2, LI Xia2,*( )
)
Received:2018-01-03
Online:2018-09-10
Published:2018-09-10
Contact:
LI Xia
E-mail:xiali@cnu.edu.cn
Supported by:摘要:
采用水热法合成了5个新的配合物[Ln2(2,2'-Hoba)6(IP)2]{Ln=Eu(1), Tb(2), 2,2'-H2oba=2,2'-联苯醚二甲酸, IP=1H-咪唑并[4,5-f][1,10]-邻菲罗啉}, [Ln2(2,2'-oba)3(IP)][Ln=Eu(3), Tb(4)]和[Eu2(2,2'-oba)3(TPP)(H2O)](5){TPP=四吡啶[2,3-a:3',2'-c:2″,3″-h:3″',2″'-j]夹二氮蒽}, 并通过X射线单晶衍射、 元素分析和红外光谱等对其结构和组成进行了确认. 配合物1和2是双核分子结构, 配合物3和4是1D链状结构, 配合物5是3D结构. 利用荧光光谱法研究了配合物1~5与NH3 的识别作用. 结果表明, NH3对配合物1~5都有一定的荧光猝灭作用, 猝灭率为82.05%~97.49%. 同时研究了配合物1与NH3和金属阳离子(Ca2+, K+, Mg2+, Na+, Zn2+, Cu2+, Cr3+, Ba2+, Al3+, Mn2+, Ni2+和Pb2+)的荧光作用. 实验结果表明, 在生理环境(pH=4~8)下, 配合物1具有良好的稳定性, NH3使配合物1的荧光性能减弱, 同时NH3对配合物1的荧光猝灭性不受pH的影响, 说明配合物1对NH3具有较好的灵敏性. 在配合物1-NH3的体系中, 加入并增加Zn2+, Al3+或Cr3+离子浓度, 在波长为430~510 nm范围内出现了新的发射宽峰, 并且在618 nm处的峰强度先增强后减弱. 这是Zn2+(Al3+或Cr3+)、 NH3与配合物1共同作用的有力证明. 配合物1作为识别NH3的荧光探针显示出较好的灵敏性, 同时一些金属阳离子对其探针行为有影响.
中图分类号:
TrendMD:
张德春, 许奇炜, 李夏. 由2,2'-联苯醚二甲酸和1H-咪唑并[4,5-f][1,10]-邻菲罗啉构筑的稀土配合物的荧光光谱及对氨的荧光传感. 高等学校化学学报, 2018, 39(12): 2611.
ZHANG Dechun,XU Qiwei,LI Xia. Lanthanide Complexes Constructed by 2,2'-Oxybis(benzoic acid) and 1H-Imidazo[4,5-f][1,10]-phenanthroline: Fluorescence and Fluorescent Sensing for NH3†. Chem. J. Chinese Universities, 2018, 39(12): 2611.
| Complex | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Empirical formula | C110H70N8O30Eu2 | C110H70N8O30Tb2 | C55H32N4O15Eu2 | C55H32N4O15Tb2 | C66H38N6O16Eu2 |
| Formula weight | 2287.66 | 2301.58 | 1292.77 | 1306.69 | 1474.94 |
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic | Monoclinic, |
| Space group | P | P | P | P | C2/c |
| a/nm | 1.15131(5) | 1.14885(5) | 1.31535(12) | 1.28091(10) | 2.84806(10) |
| b/nm | 1.38342(6) | 1.38288(6) | 1.39415(13) | 1.42179(11) | 1.40143(5) |
| c/nm | 1.70498(8) | 1.70231(8) | 1.45396(13) | 1.49624(11) | 2.97567(10) |
| α/(°) | 113.4990(10) | 113.4240(10) | 73.313(2) | 74.132(2) | 90 |
| β/(°) | 93.4370(10) | 93.4800(10) | 72.018(3) | 67.660(2) | 108.9160(10) |
| γ/(°) | 93.1820(10) | 92.9840(10) | 84.328(3) | 81.947(2) | 90 |
| V/nm3 | 2.47653(19) | 2.46845(19) | 2.4291(4) | 2.4224(3) | 11.2355(7) |
| Z | 1 | 1 | 2 | 2 | 8 |
| Dc/(Mg·m-3) | 1.534 | 1.548 | 1.767 | 1.791 | 1.744 |
| μ/mm-1 | 1.342 | 1.509 | 2.636 | 2.974 | 2.294 |
| F(000) | 1152 | 1156 | 1272 | 1280 | 5840 |
| Crystal size/mm3 | 0.234×0.139× 0.132 | 0.211×0.107× 0.067 | 0.391×0.232× 0.063 | 0.227×0.126× 0.101 | 0.300×0.204× 0.152 |
| θ Range for data collection/(°) | 2.95—25.01 | 2.95—25.01 | 2.97—25.01 | 2.90—25.01 | 2.91—27.55 |
| Limiting indices | -13≤h≤13, -16≤k≤16, -20≤l≤20 | -13≤h≤13, -16≤k≤16, -20≤l≤20 | -15≤h≤15, -16≤k≤16, -17≤l≤17 | -15≤h≤15, -16≤k≤16, -17≤l≤17 | -36≤h≤36, -17≤k≤18, -38≤l≤38 |
| Reflections collected/unique | 44087/8720 [R(int)=0.0650] | 43696/8696 [R(int)=0.0822] | 40881/8573 [R(int)=0.0930] | 39331/8520 [R(int)=0.1195] | 85920/12923 [R(int)=0.0974] |
| Data/restraints/parameters | 8720/108/718 | 8696/127/675 | 8573/0/685 | 8520/0/685 | 12923/0/812 |
| Largest difference peak and hole/(e·nm-3) | 1356 and -883 | 1353 and -779 | 1416 and -1360 | 1527 and -1000 | 1568 and -883 |
| Goodness-of-fit on F2 | 1.045 | 1.082 | 1.066 | 1.069 | 1.043 |
| Final R indices[I>2σ(I)] | R1=0.0447, wR2=0.1190 | R1=0.0522, wR2=0.1210 | R1=0.0370, wR2=0.0906 | R1=0.0462, wR2=0.0996 | R1=0.0415, wR2=0.0996 |
| R indices(all data) | R1=0.0571, wR2=0.1321 | R1=0.0780, wR2=0.1385 | R1=0.0504, wR2=0.1073 | R1=0.0794, wR2=0.1178 | R1=0.0585, wR2=0.1066 |
| CCDC No. | 1587904 | 1587905 | 1587906 | 1587909 | 1587910 |
Table 1 Crystal data and structural refinement for complexes 1—5
| Complex | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Empirical formula | C110H70N8O30Eu2 | C110H70N8O30Tb2 | C55H32N4O15Eu2 | C55H32N4O15Tb2 | C66H38N6O16Eu2 |
| Formula weight | 2287.66 | 2301.58 | 1292.77 | 1306.69 | 1474.94 |
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic | Monoclinic, |
| Space group | P | P | P | P | C2/c |
| a/nm | 1.15131(5) | 1.14885(5) | 1.31535(12) | 1.28091(10) | 2.84806(10) |
| b/nm | 1.38342(6) | 1.38288(6) | 1.39415(13) | 1.42179(11) | 1.40143(5) |
| c/nm | 1.70498(8) | 1.70231(8) | 1.45396(13) | 1.49624(11) | 2.97567(10) |
| α/(°) | 113.4990(10) | 113.4240(10) | 73.313(2) | 74.132(2) | 90 |
| β/(°) | 93.4370(10) | 93.4800(10) | 72.018(3) | 67.660(2) | 108.9160(10) |
| γ/(°) | 93.1820(10) | 92.9840(10) | 84.328(3) | 81.947(2) | 90 |
| V/nm3 | 2.47653(19) | 2.46845(19) | 2.4291(4) | 2.4224(3) | 11.2355(7) |
| Z | 1 | 1 | 2 | 2 | 8 |
| Dc/(Mg·m-3) | 1.534 | 1.548 | 1.767 | 1.791 | 1.744 |
| μ/mm-1 | 1.342 | 1.509 | 2.636 | 2.974 | 2.294 |
| F(000) | 1152 | 1156 | 1272 | 1280 | 5840 |
| Crystal size/mm3 | 0.234×0.139× 0.132 | 0.211×0.107× 0.067 | 0.391×0.232× 0.063 | 0.227×0.126× 0.101 | 0.300×0.204× 0.152 |
| θ Range for data collection/(°) | 2.95—25.01 | 2.95—25.01 | 2.97—25.01 | 2.90—25.01 | 2.91—27.55 |
| Limiting indices | -13≤h≤13, -16≤k≤16, -20≤l≤20 | -13≤h≤13, -16≤k≤16, -20≤l≤20 | -15≤h≤15, -16≤k≤16, -17≤l≤17 | -15≤h≤15, -16≤k≤16, -17≤l≤17 | -36≤h≤36, -17≤k≤18, -38≤l≤38 |
| Reflections collected/unique | 44087/8720 [R(int)=0.0650] | 43696/8696 [R(int)=0.0822] | 40881/8573 [R(int)=0.0930] | 39331/8520 [R(int)=0.1195] | 85920/12923 [R(int)=0.0974] |
| Data/restraints/parameters | 8720/108/718 | 8696/127/675 | 8573/0/685 | 8520/0/685 | 12923/0/812 |
| Largest difference peak and hole/(e·nm-3) | 1356 and -883 | 1353 and -779 | 1416 and -1360 | 1527 and -1000 | 1568 and -883 |
| Goodness-of-fit on F2 | 1.045 | 1.082 | 1.066 | 1.069 | 1.043 |
| Final R indices[I>2σ(I)] | R1=0.0447, wR2=0.1190 | R1=0.0522, wR2=0.1210 | R1=0.0370, wR2=0.0906 | R1=0.0462, wR2=0.0996 | R1=0.0415, wR2=0.0996 |
| R indices(all data) | R1=0.0571, wR2=0.1321 | R1=0.0780, wR2=0.1385 | R1=0.0504, wR2=0.1073 | R1=0.0794, wR2=0.1178 | R1=0.0585, wR2=0.1066 |
| CCDC No. | 1587904 | 1587905 | 1587906 | 1587909 | 1587910 |
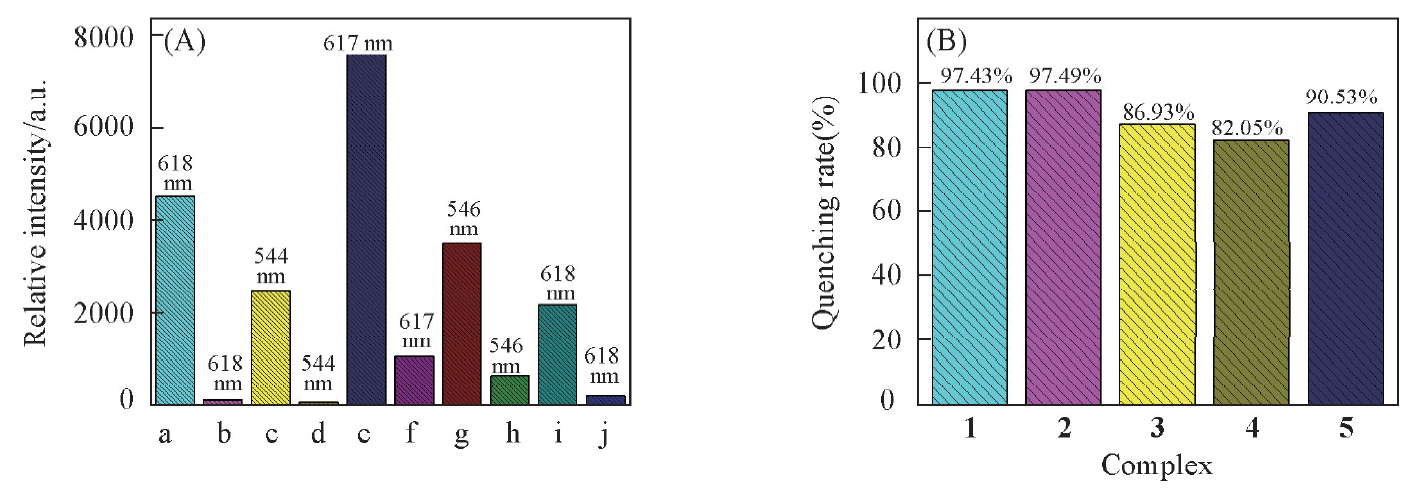
Fig.6 Fluorescence intensity(5D0→7F2 or 5D4→7F5) changes(A) and the quenching rate(B) of complexes 1—5 upon addition of NH3 at room temperaturec(NH3)=10 mmol/L, m(complex)=10 mg, V(NH3)=10 mL. (A) a. complex 1; b. complex 1+NH3; c. complex 2; d. complex 2+NH3; e. complex 3; f. compound 3+NH3; g. complex 4; h. complex 4+NH3; i. complex 5; j. complex 5+NH3.
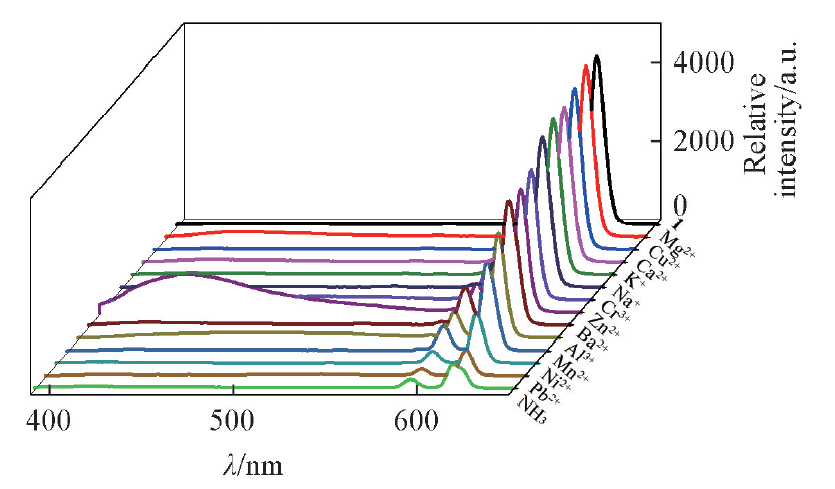
Fig.7 Fluorescence spectra changes of complex 1 upon addition of NH3 and mental ions at room temperaturec(NH3)=c(metal ion)=5 mmol/L, V=10 mL, m(complex 1)=10 mg. λex=368 nm, λem=618 nm.
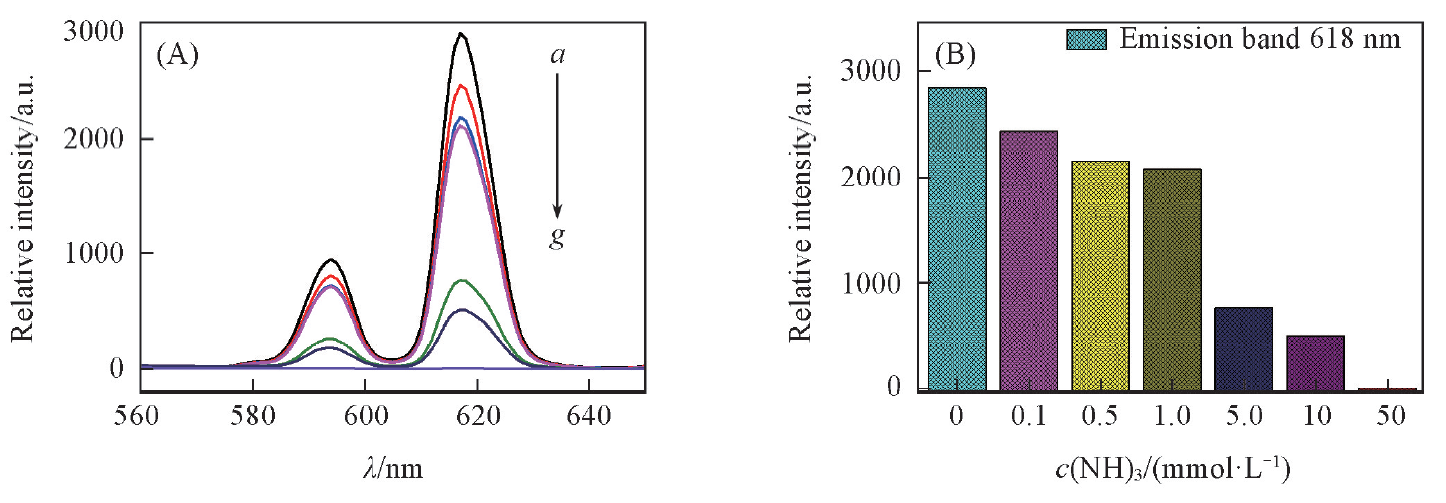
Fig.8 Effect of NH3 on the fluorescence spectra of complex 1(A), histogram of fluorescence intensity and NH3 concentration(B)c(NH3)=0, 0.1, 0.5, 1.0, 5.0, 10, 50 mmol/L(a—g), V(NH3)=10 mL, m(complex 1)=10 mg, T=25 ℃, λex=368 nm, λem=618 nm.
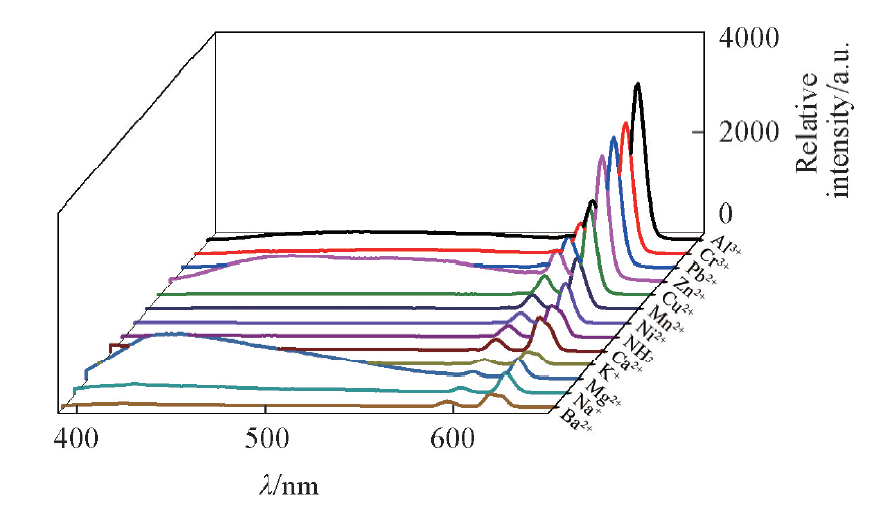
Fig.10 Emission spectra of complex 1-NH3 in the presence of different metal ionsc(NH3)=c(metal ion)=5 mmol/L, V=10 mL, m(complex 1)=10 mg, T=25 ℃, λex=368 nm, λem=618 nm.
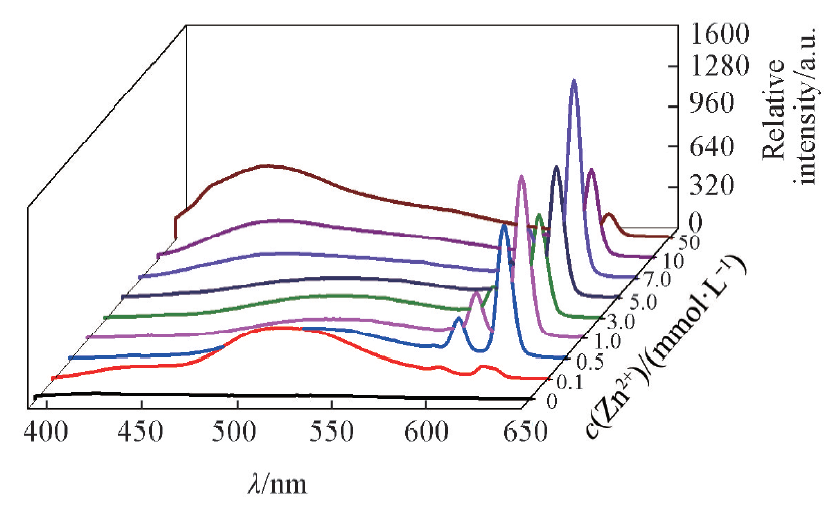
Fig.11 Effect of Zn2+ on the fluorescence spectra of complex 1-NH3c(Zn2+)=0—50 mmol/L, c(NH3)=20 mmol/L, V(Zn2+)=10 mL, m(complex 1)=10 mg, T=25 ℃, λex=368 nm, λem=618 nm.
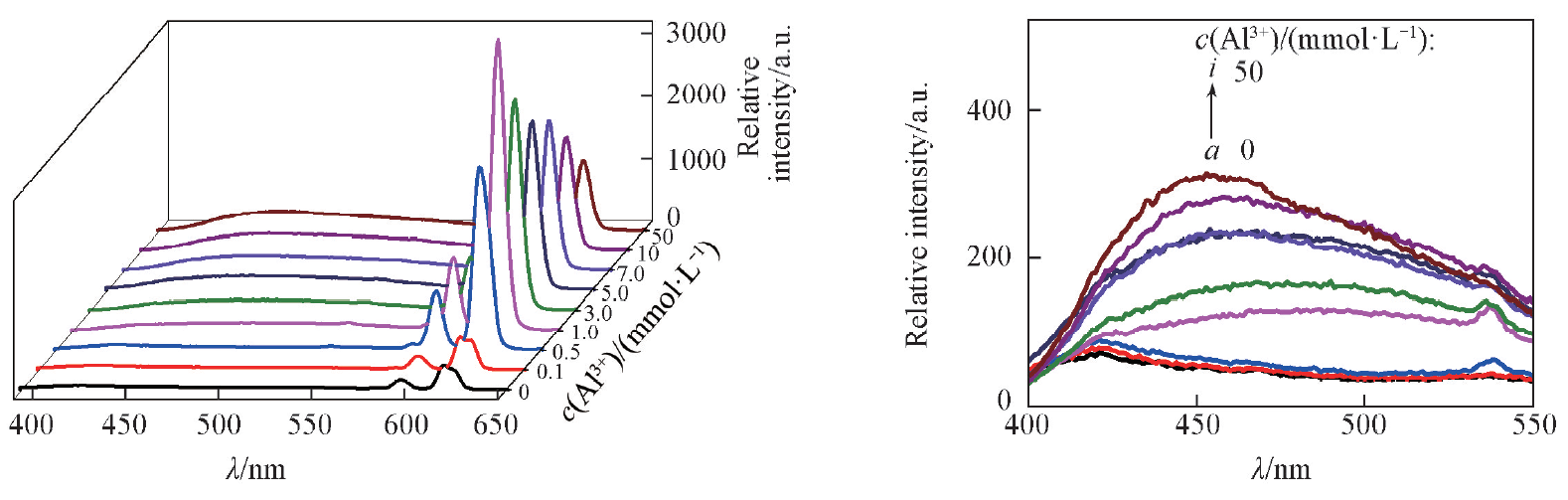
Fig.12 Effect of Al3+ on the fluorescence spectra of complex 1-NH3 c(Al3+)=0—50 mmol/L, c(NH3)=5 mmol/L, V=10 mL, m(complex 1)=10 mg, T=25 ℃, λex=368 nm, λem=618 nm.
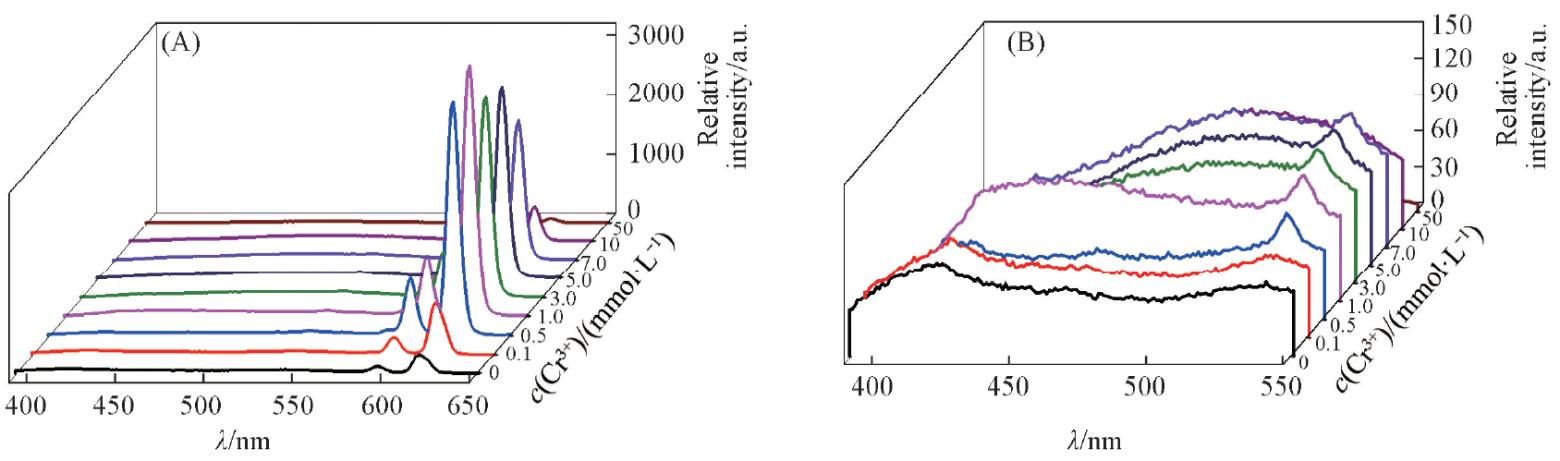
Fig.13 Effect of Cr3+ on the fluorescence spectra of complex 1-NH3c(Cr3+)=0—50 mmol/L, c(NH3)=5 mmol/L, V=10 mL, m(complex 1)=10 mg, T=25 ℃, λex=368 nm, λem=618 nm.
| [1] | Rush C.M., Lyda S.D., Mycopathologia,1982, 79, 147—152 |
| [2] | Li R.J., Liang J.X., Lv B.W., Mo J.Q., Zhao D.M., Contemp.Med.,2014, 20(15), 41—42 |
| (李荣杰, 梁俊雄, 吕博文, 莫俊强, 赵德明. 当代医学, 2014, 20(15), 41—42) | |
| [3] | Li X.G., Yu Y.G., Wang L.Y., Guo X., Wang L.M., Int. J. Lab. Med.,2012, 33(11), 1340—1342 |
| (李晓光, 于永光, 王丽艳, 郭欣, 王连明. 国际检验医学杂志, 2012, 33(11), 1340—1342) | |
| [4] | Yang Y.L., Chin. J. Med., 2006, 41(3), 18—21 |
| (杨艳玲. 中国医刊, 2006, 41#(3), 18—21) | |
| [5] | Sarıoğlan A., Durak-Çetin Y., Okutan H., Akgün F., Chem. Eng. Sci.,2017, 171, 440—450 |
| [6] | Zhang Y., Luo X.G., He K., Huo D.Q., Liu J., Liu P., Shi X.J., Hou C.J., Water, Air, & Soil Poll.,2012, 223, 2969—2977 |
| [7] | Luo X.G., Liu P., Hou C.J., Huo D.Q., Dong J.L., Fa H.B., Yang M., Rev. Sci. Instrum.,2010, 81, 105—113 |
| [8] | Ling T.L., Ahmad M., Yook H.L., Anal. Methods,2013, 5 , 6709—6714 |
| [9] | Shingaya Y., Kubo H., Ito M., Surf. Sci.,1999, 427/428, 173—178 |
| [10] | Oudenhoven J. F.M., Knoben W., van Schaijk R., Proced. Eng.,2015, 120, 983—986 |
| [11] | Katz M.J., Ramnial T., Yu H.Z., Leznoff D.B., J. Am. Chem. Soc.,2008, 130, 10662—10673 |
| [12] | Kim S.J., Hwang I.S., Kang Y.C., Lee J.H., Sensors,2011, 11, 10603—10614 |
| [13] | Shen C.Y., Huang H.C., Hwang R.C., Sensor. Actuat. A: Physical,2008, 147, 464—469 |
| [14] | Chen W.H., Chen S.M., Hung C.I., Sci. Total Environ.,2013, 444, 336—346 |
| [15] | Alvi M.A., Al-Ghamdi A.A., Khan S.A., Appl. Phys. A Mater.,2017, 123(3), 1—5 |
| [16] | Huang W., Besar K., LeCover R, Rule A. M., Breysse P.N., Katz H.E., J. Am. Chem. Soc.,2012, 134, 14650—14653 |
| [17] | Sun Y.Q., Liu Q., Zhou L.L., Chen Y.P., Cryst. Eng. Comm.,2014, 16(19), 3986—3993 |
| [18] | Wang L., Zhu X., Tang X., Wu C., Zhou Z., Sun C., Deng S.L., Ai H., Gao J., Chem. Commun.,2015, 51, 4390—4393 |
| [19] | Edelmann F.T., Chem. Soc. Rev., 2012, 41, 7657—7672 |
| [20] | Yan L., Ye Z., Peng C., Zhang S., Tetrahedron,2012, 68, 2725—2727 |
| [21] | Liu D., Lang J.P., Abrahams B.F., J. Am. Chem. Soc.,2011, 133(29), 11042—11045 |
| [22] | Liu Z., He W., Guo Z., Chem. Soc. Rev.,2013, 42, 1568—1600 |
| [23] | Zhao H.Q., Yang S.P., Ding N.N., Qin L., Qiu G.H., Chen J.X., Zhang W.H., Chen W.H., Hor T. S.A., Dalton Trans.,2016, 45, 5092—5100 |
| [24] | Qin J.S., Du D. Y., Li W. L., Zhang J. P., Li S. L., Su Z. M., Wang X. L., Xu Q., Shao K. Z., Lan Y. Q., Chem. Sci., 2012, 3, 2114—2118 |
| [25] | Wang H.N., Meng X., Yang G.S., Wang X.L., Shao K.Z., Su Z.M., Wang C.G., Chem. Commun.,2011, 47, 7128—7130 |
| [26] | Zhang D.C., Ma D., Li X., J. Coord. Chem.,2017, 70(18), 3233—3251 |
| [27] | Zhang D.C., Zhou X., Li X., Spectrosc. Spect. Anal.,2016, 36(9), 2841—2845 |
| (张德春, 周鑫, 李夏. 光谱学与光谱分析, 2016, 36(9), 2841—2845) | |
| [28] | Sun Y.Q., Wan F., Li X.X., Lin J., Wu T., Zheng S.T., Bu X., Chem. Commun.,2016, 52, 10125—10128 |
| [29] | Dong G.Y., Li R., Fan T.T., Li J.J., Li X., Chem. J. Chinese Universities,2016, 37(8), 1421—1429 |
| (董高云, 李睿, 樊婷婷, 李佳佳, 李夏. 高等学校化学学报, 2016, 37(8), 1421—1429) | |
| [30] | Chen H., Zhao X., Gao H., Zhu H.D., Jiang L.H., Ling Q.D., Chem. J. Chinese Universities,2015, 36(1), 41—47 |
| (陈鸿, 赵璇, 高慧, 朱海娣, 姜丽红, 凌启淡. 高等学校化学学报, 2015, 36(1), 41—47) | |
| [31] | Sheldrick G.M., SHELXS-97, Program for Crystal Structure Refinement, University of Götingen,Götingen, 1997 |
| [32] | Sheldrick G.M., SHELXL-97, Program for Crystal Straeture Solution, University of Göttingen, Götingen, 1997 |
| [1] | 刘晓磊, 陆永强, 游淇, 刘国辉, 姚伟, 胡日茗, 闫纪宪, 崔玉, 杨小凤, 孙国新, 蒋绪川. 基于3-羟基沙利度胺的比率型荧光探针对过氧化氢的检测[J]. 高等学校化学学报, 2022, 43(6): 20220070. |
| [2] | 蒋小康, 周琦, 周恒为. Gd2ZnTiO6∶Dy3+, Eu3+单基质白光荧光粉的制备与发光性能[J]. 高等学校化学学报, 2022, 43(6): 20220029. |
| [3] | 王君旸, 刘争, 张茜, 孙春燕, 李红霞. DNA银纳米簇在功能核酸荧光生物传感器中的应用[J]. 高等学校化学学报, 2022, 43(6): 20220010. |
| [4] | 鲁聪, 李振华, 刘金露, 华佳, 李光华, 施展, 冯守华. 一种新的镧系金属有机骨架材料的合成、 结构及荧光检测性质[J]. 高等学校化学学报, 2022, 43(6): 20220037. |
| [5] | 赵永梅, 穆叶舒, 洪琛, 罗稳, 田智勇. 双萘酰亚胺衍生物用于检测水溶液中的苦味酸[J]. 高等学校化学学报, 2022, 43(3): 20210765. |
| [6] | 李巧, 赵洋, 王恩举. 基于芳叉丙二腈的高活性迈克尔系统的吸湿反应及荧光性质[J]. 高等学校化学学报, 2022, 43(3): 20210690. |
| [7] | 周永辉, 李尧, 吴雨轩, 田晶, 徐龙权, 费旭. 一种新型光致发光自愈合水凝胶的合成[J]. 高等学校化学学报, 2022, 43(2): 20210606. |
| [8] | 田雪琴, 莫争, 丁鑫, 武鹏彦, 王雨, 王健. 方胺功能化荧光金属-有机框架材料的制备及对组氨酸的识别研究[J]. 高等学校化学学报, 2022, 43(2): 20210589. |
| [9] | 唐倩, 但飞君, 郭涛, 兰海闯. 喹啉酮-香豆素类Hg2+比色荧光探针的合成及应用[J]. 高等学校化学学报, 2022, 43(2): 20210660. |
| [10] | 伍泽鑫, 朱渊杰, 王泓中, 王均安, 贺英. 甲基修饰的咔唑/二苯砜基AIE-TADF蓝光材料及其OLED器件[J]. 高等学校化学学报, 2022, 43(11): 20220371. |
| [11] | 王迪, 钟克利, 汤立军, 侯淑华, 吕春欣. 席夫碱共价有机框架的合成及对I ‒ 的识别[J]. 高等学校化学学报, 2022, 43(10): 20220115. |
| [12] | 刘苗, 刘瑞波, 刘巴蒂, 钱鹰. 溶酶体靶向吲哚氟硼二吡咯光敏剂的合成、 双光子荧光成像及光动力治疗[J]. 高等学校化学学报, 2022, 43(10): 20220326. |
| [13] | 李文, 乔珺一, 刘鑫垚, 刘云凌. 含萘基团的锆金属有机骨架材料对水中硝基芳烃爆炸物的荧光检测性能[J]. 高等学校化学学报, 2022, 43(1): 20210654. |
| [14] | 马鉴新, 刘晓东, 徐娜, 刘国成, 王秀丽. 一种具有发光传感、 安培传感和染料吸附性能的多功能Zn(II)配位聚合物[J]. 高等学校化学学报, 2022, 43(1): 20210585. |
| [15] | 吴季, 张浩, 骆昱晖, 耿吴越, 兰亚乾. 一种具有荧光性质的阳离子Ga⁃MOF用于Fe3+和硝基化合物识别[J]. 高等学校化学学报, 2022, 43(1): 20210617. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||