

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (10): 3151.doi: 10.7503/cjcu20210372
巩珊珊1,2, 吴彤1, 王官格1, 黄擎1,2( ), 苏岳锋1,2, 吴锋1,2
), 苏岳锋1,2, 吴锋1,2
收稿日期:2021-06-01
出版日期:2021-10-10
发布日期:2021-10-10
通讯作者:
黄擎
E-mail:huangqing3121@sina.com
基金资助:
GONG Shanshan1,2, WU Tong1, WANG Guange1, HUANG Qing1,2( ), SU Yuefeng1,2, WU Feng1,2
), SU Yuefeng1,2, WU Feng1,2
Received:2021-06-01
Online:2021-10-10
Published:2021-10-10
Contact:
HUANG Qing
E-mail:huangqing3121@sina.com
Supported by:摘要:
针对废旧锂离子电池(LIBs)回收过程中产生的二次污染及高能耗等问题, 提出了一种绿色高效浸出废旧LIBs正极材料中有价金属的新方法. 以氯化胆碱和不同的氢键供体(草酸、 丙二酸、 戊二酸和苯磺酸)为原料, 合成了氯化胆碱/酸二元低共熔溶剂(DES)、 氯化胆碱/酸/水和氯化胆碱/酸/乙醇等三元DES. 通过傅里叶变换红外光谱(FTIR)和核磁共振波谱(NMR)表征了氯化胆碱和酸之间氢键的形成过程, 探究了DES中羧酸的烷基链长、 酸性大小以及添加水和乙醇组分对浸出废旧LiCoO2正极材料的影响. 研究结果表明, 羧酸烷基链长的增加会使DES的浸出能力下降; 酸的酸性大小不能作为溶解金属氧化物能力强弱的主要依据; 加入等摩尔量的水对DES的浸出效率影响较小, 而等摩尔量加入无水乙醇会影响DES的氢键结构, 对浸出结果影响较大. 筛选出氯化胆碱/苯磺酸/乙醇DES作为废旧LiCoO2绿色高效的浸出剂, Li和Co的浸出效率分别高达98.6%和95.2%.
中图分类号:
TrendMD:
巩珊珊, 吴彤, 王官格, 黄擎, 苏岳锋, 吴锋. 基于高效回收废旧锂离子电池正极材料的低共熔溶剂的筛选. 高等学校化学学报, 2021, 42(10): 3151.
GONG Shanshan, WU Tong, WANG Guange, HUANG Qing, SU Yuefeng, WU Feng. Screening of Deep Eutectic Solvent Based on Efficient Recovery of Spent Lithium⁃ion Battery Cathode Materials. Chem. J. Chinese Universities, 2021, 42(10): 3151.
| Sample | Component | Molar ratio | Mass ratio | Sample | Component | Molar ratio | Mass ratio |
|---|---|---|---|---|---|---|---|
| DES?1 | ChCl/Oxa | 1∶1 | 6.98∶4.52 | DES?7 | ChCl/Glu | 1∶1 | 6.98∶6.61 |
| DES?2 | ChCl/Oxa/H2O | 1∶1∶1 | 6.98∶4.52∶0.90 | DES?8 | ChCl/Glu/H2O | 1∶1∶1 | 6.98∶6.61∶0.90 |
| DES?3 | ChCl/Oxa/Ethanol | 1∶1∶1 | 6.98∶4.52∶2.30 | DES?9 | ChCl/Glu/Ethanol | 1∶1∶1 | 6.98∶6.61∶2.30 |
| DES?4 | ChCl/Mal | 1∶1 | 6.98∶5.20 | DES?10 | ChCl/Ben | 1∶1 | 6.98∶7.91 |
| DES?5 | ChCl/Mal/H2O | 1∶1∶1 | 6.98∶5.20∶0.90 | DES?11 | ChCl/Ben/H2O | 1∶1∶1 | 6.98∶7.91∶0.90 |
| DES?6 | ChCl/Mal/Ethanol | 1∶1∶1 | 6.98∶5.20∶2.30 | DES?12 | ChCl/Ben/Ethanol | 1∶1∶1 | 6.98∶7.91∶2.30 |
Table 1 Summary of the components for the preparation of DES
| Sample | Component | Molar ratio | Mass ratio | Sample | Component | Molar ratio | Mass ratio |
|---|---|---|---|---|---|---|---|
| DES?1 | ChCl/Oxa | 1∶1 | 6.98∶4.52 | DES?7 | ChCl/Glu | 1∶1 | 6.98∶6.61 |
| DES?2 | ChCl/Oxa/H2O | 1∶1∶1 | 6.98∶4.52∶0.90 | DES?8 | ChCl/Glu/H2O | 1∶1∶1 | 6.98∶6.61∶0.90 |
| DES?3 | ChCl/Oxa/Ethanol | 1∶1∶1 | 6.98∶4.52∶2.30 | DES?9 | ChCl/Glu/Ethanol | 1∶1∶1 | 6.98∶6.61∶2.30 |
| DES?4 | ChCl/Mal | 1∶1 | 6.98∶5.20 | DES?10 | ChCl/Ben | 1∶1 | 6.98∶7.91 |
| DES?5 | ChCl/Mal/H2O | 1∶1∶1 | 6.98∶5.20∶0.90 | DES?11 | ChCl/Ben/H2O | 1∶1∶1 | 6.98∶7.91∶0.90 |
| DES?6 | ChCl/Mal/Ethanol | 1∶1∶1 | 6.98∶5.20∶2.30 | DES?12 | ChCl/Ben/Ethanol | 1∶1∶1 | 6.98∶7.91∶2.30 |
| Sample | Appearance | δ | |
|---|---|---|---|
| DES?1 | Transparent viscous liquid | 3307.9(O―H), 1724.2(C=O), 1474.6 (―O―H), 950.0(N―CH3) | 3.79―3.84(―CH2―O―), 3.19―3.27(―N―CH2―), 2.97(―N―CH3) |
| DES?4 | Transparent viscous liquid | 2930.2(―C―CH2), 1717.2(C=O), 1476.7(―O―H), 951.6(N―CH3) | 3.93―3.97(―CH2―O―), 3.40―3.43(―N―CH2―), 3.09(―N―CH3) |
| DES?7 | Transparent liquid | 2946.3(―C―CH2), 1716.7(C=O), 1477.8(―O―H), 1147.8(―CH2―), 951.7(N―CH3) | 3.5(―N―CH2―), 3.17(―N―CH3), 2.39―2.43 (―CH2―C=O―), 1.80―1.87(―CH2―) |
| DES?10 | Transparent liquid | 2946.3(―C―CH2), 1716.7(C=O), 1477.8(―O―H), 1147.8(―CH2―), 951.7(N―CH3) | 7.66―7.68(Ar―H), 7.38―7.44(Ar―H), 3.84―3.88 (―CH2―O―), 3.29―3.32(―N―CH2―), 2.99(―N―CH3) |
Table 2 Comparison of FTIR and 1H NMR data of DES-1, DES-4, DES-7 and DES-10
| Sample | Appearance | δ | |
|---|---|---|---|
| DES?1 | Transparent viscous liquid | 3307.9(O―H), 1724.2(C=O), 1474.6 (―O―H), 950.0(N―CH3) | 3.79―3.84(―CH2―O―), 3.19―3.27(―N―CH2―), 2.97(―N―CH3) |
| DES?4 | Transparent viscous liquid | 2930.2(―C―CH2), 1717.2(C=O), 1476.7(―O―H), 951.6(N―CH3) | 3.93―3.97(―CH2―O―), 3.40―3.43(―N―CH2―), 3.09(―N―CH3) |
| DES?7 | Transparent liquid | 2946.3(―C―CH2), 1716.7(C=O), 1477.8(―O―H), 1147.8(―CH2―), 951.7(N―CH3) | 3.5(―N―CH2―), 3.17(―N―CH3), 2.39―2.43 (―CH2―C=O―), 1.80―1.87(―CH2―) |
| DES?10 | Transparent liquid | 2946.3(―C―CH2), 1716.7(C=O), 1477.8(―O―H), 1147.8(―CH2―), 951.7(N―CH3) | 7.66―7.68(Ar―H), 7.38―7.44(Ar―H), 3.84―3.88 (―CH2―O―), 3.29―3.32(―N―CH2―), 2.99(―N―CH3) |
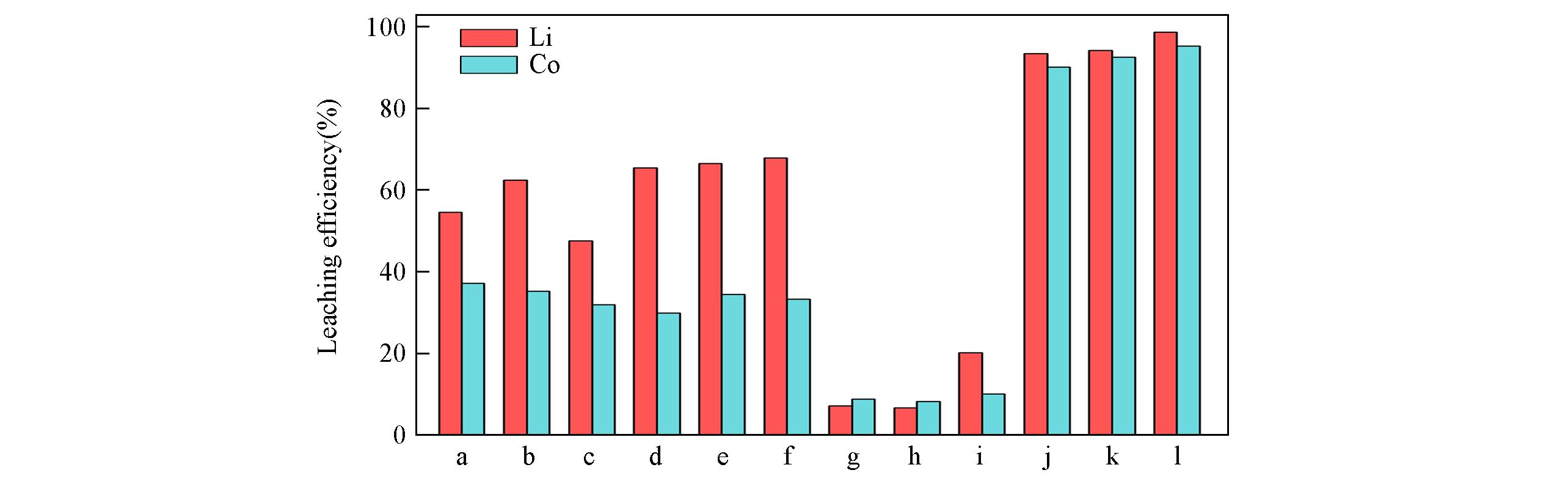
Fig.6 Leaching efficiency of spent LiCoO2 cathode materials in different DESsa. DES?1; b. DES?2; c. DES?3; d. DES?4; e. DES?5; f. DES?6; g. DES?7; h. DES?8; i. DES?9; j. DES?10; k. DES?11; l. DES?12.
| Leachate | Reductant | t/min | Temperature/℃ | Solid/Liquid ratio/(g·L-1) | Leaching efficiency of Li(%) | Leaching efficiency of Co(%) | Ref. |
|---|---|---|---|---|---|---|---|
| Citric acid(2.0 mol/L) | H2O2(2%) | 80 | 70 | 50 | 99.0 | 98.0 | [ |
| Malic acid(1.5 mol/L) | H2O2(2%) | 40 | 90 | 20 | 99.0 | 93.0 | [ |
| Succinic acid(1.5 mol/L) | H2O2(4%) | 40 | 70 | 15 | 94.7 | 99.8 | [ |
| Lactic acid(1.5 mol/L) | H2O2(0.5%) | 20 | 70 | 20 | 97.7 | 98.9 | [ |
| Phosphoric acid(1.5 mol/L) | Glucose (0.02 mol/L) | 120 | 80 | 2 | 100 | 98.0 | [ |
| Citric acid(1.5 mol/L) | Ascorbic acid (0.02 mol/L) | 360 | 80 | 2 | 100 | 80.0 | [ |
| Ascorbic acid(1.25 mol/L) | — | 20 | 70 | 25 | 98.5 | 94.8 | [ |
| Oxalic acid(1.0 mol/L) | — | 120 | 80 | 50 | 98.0 | 68.0 | [ |
ChCl/EG DES (1∶2, molar ratio) | — | 1440 | 220 | 22 | — | 94.1 | [ |
ChCl/U DES (1∶2, molar ratio) | — | 720 | 180 | 24 | 94.7 | 97.9 | [ |
ChCl/PTSA/H2O DES (1∶1∶2, molar ratio) | — | 15 | 90 | 60 | 100 | 100 | [ |
ChCl/Citric acid DES (1∶2, molar ratio) | Al(12%), Cu(24%) | 60 | 40 | 20 | 93.0 | 98.0 | [ |
ChCl/Ben/Ethanol DES (1∶1∶1, molar ratio) | — | 60 | 90 | 20 | 98.6 | 95.2 | This work |
Table 3 Representative organic acids and DES for recycling spent LIBs*
| Leachate | Reductant | t/min | Temperature/℃ | Solid/Liquid ratio/(g·L-1) | Leaching efficiency of Li(%) | Leaching efficiency of Co(%) | Ref. |
|---|---|---|---|---|---|---|---|
| Citric acid(2.0 mol/L) | H2O2(2%) | 80 | 70 | 50 | 99.0 | 98.0 | [ |
| Malic acid(1.5 mol/L) | H2O2(2%) | 40 | 90 | 20 | 99.0 | 93.0 | [ |
| Succinic acid(1.5 mol/L) | H2O2(4%) | 40 | 70 | 15 | 94.7 | 99.8 | [ |
| Lactic acid(1.5 mol/L) | H2O2(0.5%) | 20 | 70 | 20 | 97.7 | 98.9 | [ |
| Phosphoric acid(1.5 mol/L) | Glucose (0.02 mol/L) | 120 | 80 | 2 | 100 | 98.0 | [ |
| Citric acid(1.5 mol/L) | Ascorbic acid (0.02 mol/L) | 360 | 80 | 2 | 100 | 80.0 | [ |
| Ascorbic acid(1.25 mol/L) | — | 20 | 70 | 25 | 98.5 | 94.8 | [ |
| Oxalic acid(1.0 mol/L) | — | 120 | 80 | 50 | 98.0 | 68.0 | [ |
ChCl/EG DES (1∶2, molar ratio) | — | 1440 | 220 | 22 | — | 94.1 | [ |
ChCl/U DES (1∶2, molar ratio) | — | 720 | 180 | 24 | 94.7 | 97.9 | [ |
ChCl/PTSA/H2O DES (1∶1∶2, molar ratio) | — | 15 | 90 | 60 | 100 | 100 | [ |
ChCl/Citric acid DES (1∶2, molar ratio) | Al(12%), Cu(24%) | 60 | 40 | 20 | 93.0 | 98.0 | [ |
ChCl/Ben/Ethanol DES (1∶1∶1, molar ratio) | — | 60 | 90 | 20 | 98.6 | 95.2 | This work |
| 1 | Zhao Y. L., Yuan X. Z., Jiang L. B., Wen J., Wang H., Guan R. P., Zhang J. J., Zeng G. M., Chem. Eng. J., 2020, 383, 123089 |
| 2 | Fan E. S., Yang J. B., Huang Y. X., Lin J., Arshad. F., Wu F., Li L., Chen R. J., ACS Appl. Energy Mater., 2020, 3(9), 8532—8542 |
| 3 | Fan E. S., Li L., Wang Z. P., Lin J., Huang Y. Y., Yao Y., Chen R. J., Wu F., Chem. Rev., 2020, 120(14), 7020—7063 |
| 4 | Boxall N. J., Adamek N., Cheng K. Y., Haque N., Bruckard W., Kaksonen A. H., Waste Manage., 2018, 74, 435—445 |
| 5 | Chen X. P., Ma H. R., Luo C. B., Zhou T., J. Hazard. Mater., 2017, 326, 77—86 |
| 6 | Natarajan S., Aravindan V., Adv. Energy Mater., 2018, 8(33), 1802303 |
| 7 | Chitre A., Freake D., Lander L., Edge J., Titirici M. M., Batteries Supercaps., 2020, 3(11), 1125 |
| 8 | Xu P. P., Dai Q., Gao H. P., Liu H. D., Zhang M. H., Li M. Q., Chen Y., An K., Meng Y. S., Liu P., Li Y. R., Spangenberger J. S., Gaines L., Lu J., Chen Z., Joule, 2020, 4(12), 2609—2626 |
| 9 | Wang M. M., Tan Q. Y., Li J. H., Environ. Sci. Technol., 2018, 52(22), 13136—13143 |
| 10 | Wang M. M., Zhang C. C., Zhang F. S., Waste Manage., 2017, 67, 232—239 |
| 11 | Ciez R. E., Whitacre J. F., Nat. Sustain., 2019, 2(2), 148—156 |
| 12 | Gao W. F., Zhang X. H., Zheng X. H., Lin X., Cao H. B., Zhang Y., Sun Z., Environ. Sci. Technol., 2017, 51(3), 1662—1669 |
| 13 | Meng Q., Zhang Y. J., Dong P., Waste Manage., 2017, 64, 214—218 |
| 14 | Yang J., Qin J. T., Li F. C., Jiang L. X., Lai Y. Q., Liu F. Y., Jia M., J. Cent. South Univ.(Sci. Technol.), 2020, 51(12), 3261—3278(杨健, 秦吉涛, 李芳成, 蒋良兴, 赖延清, 刘芳洋, 贾明. 中南大学学报(自然科学版), 2020, 51(12), 3261—3278) |
| 15 | Li L. L., Cao L. J., Mai Y. X., Men Y. F., Yang W., Chen S. Z., Energy Storage Sci. Technol., 2020, 9(6), 1641—1650(李林林, 曹林娟, 麦永雄, 门一飞, 杨伟, 陈胜洲. 储能科学与技术, 2020, 9(6), 1641—1650) |
| 16 | Billy E., Joulié M., Laucournet R., Boulineau A., De V. E., Meyer D., ACS Appl. Mater. Interfaces, 2018, 10(19), 16424—16435 |
| 17 | Abbott A. P., Capper G., Davies D. L., Rasheed R. K., Tambyrajah V., Chem. Commun., 2003, (1), 70—71 |
| 18 | Gurkan B. E., Maginn E. J., Pentzer E. B., J. Phys. Chem. B,2020, 124(50), 11313—11315 |
| 19 | Hansen B. B., Spittle S., Chen B., Poe D., Zhang Y., Klein J. M., Horton A., Adhikari L., Zelovich T., Doherty B., Gurkan B., Maginn E. J., Ragauskas A., Dadmun M., Zawodzinski T. A., Baker G. A., Tuckerman M. E., Savinell R. F., Sangoro J. R., Chem. Rev., 2021, 121(3), 1232—1285 |
| 20 | Paiva A., Craveiro R., Aroso I., Martins M., Reis R. L., Duarte A. R., ACS Sustainable Chem. Eng., 2014, 2(5), 1063—1071 |
| 21 | Carriazo D., Serrano M. C., Gutierrez M. C., Ferrer M. L., Del M. F., Chem. Soc. Rev., 2012, 41(14), 4996—5014 |
| 22 | Abbott A. P., Boothby D., Capper G., Davies D. L., Rasheed R. K., J. Am. Chem. Soc., 2004, 126(29), 9142—9147 |
| 23 | Tran M. K., Rodrigues M. F., Kato K., Babu G., Ajayan P. M., Nat. Energy, 2019, 4(4), 339—345 |
| 24 | Roldán⁃Ruiz M. J., Ferrer M. L., Gutiérrez M. C., Monte F. D., ACS Sustainable Chem. Eng., 2020, 8(14), 5437—5445 |
| 25 | Wang S. B., Zhang Z. T., Lu Z. G., Xu Z. H., Green Chem., 2020, 22(14), 4473—4482 |
| 26 | Sirviö J. A., Visanko M., Liimatainen H., Biomacromolecules, 2016, 17(9), 3025—3032 |
| 27 | Hammond O. S., Bowron D. T., Jackson A. J., Arnold T., Sanchez⁃Fernandez A., Tsapatsaris N., Garcia S. V., Edler K. J., J. Phys. Chem. B,2017, 121(31), 7473—7483 |
| 28 | Yu M., Zhang Z. H., Xue F., Yang B., Guo G. H., Qiu J. H., Sep. Purif. Technol., 2019, 215, 398—402 |
| 29 | Rodriguez R. N., Machiels L., Binnemans K., ACS Sustainable Chem. Eng., 2019, 7(4), 3940—3948 |
| 30 | Florindo C., Oliveira F. S., Rebelo L. P. N., Fernandes A. M., Marrucho I. M., ACS Sustainable Chem. Eng., 2014, 2(10), 2416—2425 |
| 31 | Delgado⁃Mellado N., Larriba M., Navarro P., Rigual V., Ayuso M., García J., Rodríguez F., J. Mol. Liq., 2018, 260, 37—43 |
| 32 | Gontrani L., Plechkova N. V., Bonomo M., ACS Sustainable Chem. Eng., 2019, 7(14), 12536—12543 |
| 33 | Peeters N., Binnemans K., Riaño S., Green Chem., 2020, 22(13), 4210—4221 |
| 34 | Zhao J. J., Zhang B. L., Xie H. W., Qu J. K., Qu X., Xing P. F., Yin H. Y., Environ Res., 2020, 181, 108803 |
| 35 | Fu Y. P., He Y. Q., Chen H. C., Ye C. L., Lu Q. C., Li R. N., Xie W. N., Wang J., J. Ind. Eng. Chem.,2019, 79, 154—162 |
| 36 | Chen X. P., Luo C. B., Zhang J. X., Kong J. R., Zhou T., ACS Sustainable Chem. Eng., 2015, 3(12), 3104—3113 |
| 37 | Li L., Dunn J. B., Zhang X. X., Gaines L., Chen R. J., Wu F., Amine K., J. Power Sources, 2013, 233, 180—189 |
| 38 | Li L., Qu W. J., Zhang X. X., Lu J., Chen R. J., Wu F., Amine K., J. Power Sources, 2015, 282, 544—551 |
| 39 | Li L., Fan E. S., Guan Y. B., Zhang X. X., Xue Q., Wei L., Wu F., Chen R. J., ACS Sustainable Chem. Eng., 2017, 5(6), 5224—5233 |
| 40 | Nayaka G. P., Manjanna J., Pai K. V., Vadavi R., Keny S. J., Ripathi V. S., Hydrometallurgy, 2015, 151, 73—77 |
| 41 | Li L., Lu J., Ren Y., Zhang X. X., Chen R. J., Wu F., Amine K., J. Power Sources, 2012, 218, 21—27 |
| 42 | Sun L., Qiu K. Q., Waste Manage., 2012, 32(8), 1575—1582 |
| [1] | 闵婧, 王力彦. 利用三中心氢键限制芳酰胺构象的核磁共振氢谱分析[J]. 高等学校化学学报, 2022, 43(6): 20220084. |
| [2] | 张勇, 许俊, 鲍雨, 崔树勋. 非极性有机溶剂对分子内氢键弱化程度的单分子力谱定量研究[J]. 高等学校化学学报, 2022, 43(4): 20210863. |
| [3] | 崔韶丽, 张维佳, 邵学广, 蔡文生. 自由能计算揭示苏氨酸对抗冻蛋白与冰晶结合能力的影响[J]. 高等学校化学学报, 2022, 43(3): 20210838. |
| [4] | 胡波, 朱昊辰. 双层氧化石墨烯纳米体系中受限水的介电常数[J]. 高等学校化学学报, 2022, 43(2): 20210614. |
| [5] | 高慧玲, 曹珍珍, 顾芳, 王海军. 氢键型水凝胶自修复行为的Monte Carlo模拟[J]. 高等学校化学学报, 2022, 43(11): 20220482. |
| [6] | 王乐, 秦刘磊, 刘洋, 任丽, 徐慧婷, 刘尊奇. 一维链状氢键型甘氨酸超分子化合物[(Gly)2+ (18-crown-6)2(MnCl4)2‒]的合成、 结构及介电性质[J]. 高等学校化学学报, 2021, 42(3): 691. |
| [7] | 倪卿盛, 杜淼, 单国荣, 宋义虎, 吴子良, 郑强. 一维粒子对聚乙烯醇水溶液流变行为的调控[J]. 高等学校化学学报, 2021, 42(12): 3738. |
| [8] | 李晓蕾, 孙云娇, 唐颖, 王长生. 醇及脱氧核糖与水分子间三体作用强度的快速准确计算[J]. 高等学校化学学报, 2021, 42(12): 3664. |
| [9] | 白兰, 翟磊, 王畅鸥, 何民辉, 莫松, 范琳. 含酰胺结构超低膨胀聚酰亚胺薄膜的热膨胀行为[J]. 高等学校化学学报, 2020, 41(4): 795. |
| [10] | 秦刘磊,刘洋,关小琴,郑晓媛,张子钰,刘尊奇. 无机-有机杂化化合物[(H2DABCO)CuCl4]·H2O的合成及开关型介电性质[J]. 高等学校化学学报, 2020, 41(1): 70. |
| [11] | 许炎, 刘翠, 韩成娟, 潘明玉, 孙照琦, 韩冰玉, 杨忠志. 鸟嘌呤与氨基酸残基体系的极化力场[J]. 高等学校化学学报, 2019, 40(2): 288. |
| [12] | 徐宇, 花儿. 烷基乙二胺-CF3CO2型质子化离子液体的分子间氢键作用[J]. 高等学校化学学报, 2018, 39(9): 1954. |
| [13] | 欧阳顺利, 张明哲, 张永朝, 胡庆成, 魏海燕, 吴楠楠, 黄保坤. 三元水溶液体系中氢键作用与分子结构的拉曼光谱研究[J]. 高等学校化学学报, 2018, 39(4): 758. |
| [14] | 夏萌, 彭雄伟, 高红飞, 严超, 陈慧茹, 程晓红. 巴比妥酸楔形棒状液晶分子的合成、性质及与三嗪衍生物的氢键识别组装体[J]. 高等学校化学学报, 2017, 38(7): 1203. |
| [15] | 韩冰玉, 李月, 刘翠. 氨基酸侧链与氧化鸟嘌呤碱基对体系的氢键作用[J]. 高等学校化学学报, 2017, 38(6): 1068. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||