

高等学校化学学报 ›› 2019, Vol. 40 ›› Issue (3): 489.doi: 10.7503/cjcu20180596
张克杰1,2( ), 李宇1, 夏源1, 韩烁1, 曹静1, 王瀚漾1, 罗文韬1, 周志萍3
), 李宇1, 夏源1, 韩烁1, 曹静1, 王瀚漾1, 罗文韬1, 周志萍3
收稿日期:2019-08-27
出版日期:2019-01-24
发布日期:2019-01-24
作者简介:联系人简介: 张克杰, 女, 博士, 副教授, 主要从事无机纳米复合材料研究. E-mail:
基金资助:
ZHANG Kejie1,2,*( ), LI Yu1, XIA Yuan1, HAN Shuo1, CAO Jing1, WANG Hanyang1, LUO Wentao1, ZHOU Zhiping3
), LI Yu1, XIA Yuan1, HAN Shuo1, CAO Jing1, WANG Hanyang1, LUO Wentao1, ZHOU Zhiping3
Received:2019-08-27
Online:2019-01-24
Published:2019-01-24
Contact:
ZHANG Kejie
E-mail:zhangkejie2003@163.com
Supported by:摘要:
以乙酸镉、 2-巯基苯并噻唑、 硫化钠和氯化铜为原料, 依次利用液相热分解与离子吸附法, 改变CdS晶化时间及含量, 制备了4种CdS/CuS纳米复合材料. 研究结果表明: CdS/CuS纳米复合材料呈类球形核壳结构, 改变CdS晶化时间可以控制CdS/CuS纳米复合材料粒径大小; CdS的晶化时间为10 min, CdS与CuS摩尔比为4∶1的纳米复合材料光催化活性最佳, 25 min内对RhB和MB的降解效率均达到99%.
中图分类号:
TrendMD:
张克杰, 李宇, 夏源, 韩烁, 曹静, 王瀚漾, 罗文韬, 周志萍. 核壳结构CdS/CuS纳米复合材料的制备及光催化性能. 高等学校化学学报, 2019, 40(3): 489.
ZHANG Kejie,LI Yu,XIA Yuan,HAN Shuo,CAO Jing,WANG Hanyang,LUO Wentao,ZHOU Zhiping. Synthesis and Photocatalytic Performance of CdS/CuS Core-shell Nanocomposites†. Chem. J. Chinese Universities, 2019, 40(3): 489.
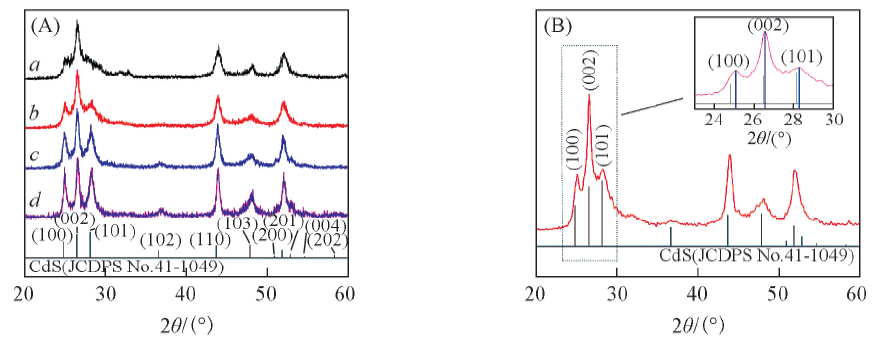
Fig.1 XRD patterns of CdS/CuS samples(A) a. CdS(10 min)/CuS(3∶1); b. CdS(10 min)/CuS(4∶1); c. CdS(10 min)/CuS(5∶1);d. CdS(1 h)/CuS(4∶1). (B) CdS(10 min)/CuS(4∶1).
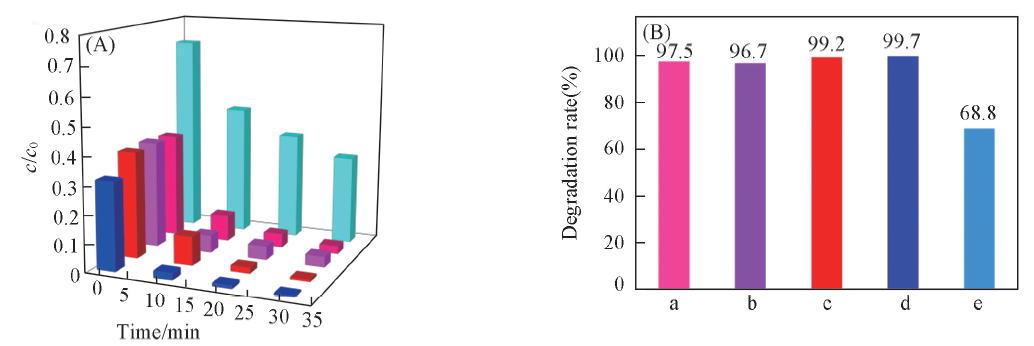
Fig.9 Degradation profiles of different dyes in the presence of different photocatalysts(A) and the photocatalytic efficiency of different photocatalysts for different dyes within 35 min(B)(A)CdS(1 h)/CuS(4∶1), MB;CdS(10 min)/CuS(3∶1), RhB; CdS(10 min)/CuS(5∶1), RhB;CdS(10 min)/CuS(4∶1), RhB;CdS(10 min)/CuS(4∶1), MB. (B) a. CdS(10 min)/CuS(3∶1), RhB; b. CdS(10 min)/CuS(5∶1), RhB; c. CdS(10 min)/CuS(4∶1), RhB; d. CdS(10 min)/CuS(4∶1), MB; e. CdS(1 h)/CuS(4∶1), MB.
| Sample | Dye | First-order kinetic equation | R2 |
|---|---|---|---|
| CdS(10 min)/CuS(3∶1) | RhB | -ln(c/c0)=0.081t+1.437 | 0.878 |
| CdS(10 min)/CuS(4∶1) | RhB | -ln(c/c0)=0.157t+0.757 | 0.998 |
| CdS(10 min)/CuS(5∶1) | RhB | -ln(c/c0)=0.025t+2.489 | 0.995 |
| CdS(10 min)/CuS(4∶1) | MB | -ln(c/c0)=0.115t+1.871 | 0.954 |
| CdS(1 h)/CuS(4∶1) | MB | -ln(c/c0)=0.020t+0.488 | 0.947 |
Table 1 Kinetic equation of dyes degradation by different photocatalysts
| Sample | Dye | First-order kinetic equation | R2 |
|---|---|---|---|
| CdS(10 min)/CuS(3∶1) | RhB | -ln(c/c0)=0.081t+1.437 | 0.878 |
| CdS(10 min)/CuS(4∶1) | RhB | -ln(c/c0)=0.157t+0.757 | 0.998 |
| CdS(10 min)/CuS(5∶1) | RhB | -ln(c/c0)=0.025t+2.489 | 0.995 |
| CdS(10 min)/CuS(4∶1) | MB | -ln(c/c0)=0.115t+1.871 | 0.954 |
| CdS(1 h)/CuS(4∶1) | MB | -ln(c/c0)=0.020t+0.488 | 0.947 |
| [1] | Chen H. M., Chen C. K., Liu R. S., Zhang L., Zhang J., Wilkinson D. P., Chem. Soc. Rev., 2012, 41(17), 5654—5671 |
| [2] | Ramasamy K., Sims H., Butler W.H.., Gupta A.,J. Am. Chem. Soc., 2014, 136(4), 1587—1598 |
| [3] | Liu B. W., Zeng H. Y., Zhang M. J., Fan Y. H., Guo G. C., Huang J. S., Dong Z. C., Inorg. Chem., 2015, 54(3), 976—981 |
| [4] | Luo M., Liu Y., Hu J., Liu H., Li J., ACS Appl.Mater. Interfaces, 2012, 4(3), 1813—1821 |
| [5] | Han J. H., Kwak M., Kim Y., Cheon J., Chem. Rev., 2019, 118(13), 6151—6188 |
| [6] | Deng X., Wang C., Yang H., Shao M., Zhang S., Wang X., Ding M., Huang J., Xu X., Sci. Rep.-UK, 2017, 7(1), 3877—3888 |
| [7] | Yang J., Wang J., Li X., Wang D., Song H., Catal. Sci. Technol., 2016, 6(12), 4525—4534 |
| [8] | Sarkar A., Ghosh A.B.., Saha N., Srivastava D. N., Paul P., Adhikary B.,J. Colloid Interface Sci., 2016, 483, 49—59 |
| [9] | Wang L., Wen M., Wang W., Momuinou N., Wang Z., Li S., J. Alloy Compd., 2016, 683, 318—328 |
| [10] | Wu G., Xiao L., Gu W., Shi W., Jiang D., Liu C., RSC Adv., 2016, 6(24), 19878—19886 |
| [11] | Han H., Kim K. M., Choi H., Ali G., Chung K. Y., Hong Y.-R., Choi J., Kwon J., Lee S. W., Lee J. W., Ryu J. H., Song T., Mhin S., ACS Catal., 2019, 8(5), 4091—4102 |
| [12] | Song J., Zhao H., Sun R., Li X., Sun D., Energ. Environ. Sci., 2017, 10(1), 225—235 |
| [13] | Zhou S., Yin L., J. Alloy Compd., 2017, 691, 1040—1048 |
| [14] | Habisreutinger S. N., Schmidt-Mende L., Stolarczyk J. K., Angew. Chem. Int. Ed. Engl., 2013, 52(29), 7372—7408 |
| [15] | Liu X., Inagaki S., Gong J., Angew. Chem. Int. Ed. Engl., 2016, 55(48), 14924—14950 |
| [16] | Nakajima T., Tamaki Y., Ueno K., Kato E., Nishikawa T., Ohkubo K., Yamazaki Y., Morimoto T., Ishitani O., J. Am. Chem. Soc., 2016, 138(42), 13818—13821 |
| [17] | Bu Y., Chen Z., Li W., Yu J., ACS Appl.Mater. Interfaces, 2013, 5(11), 5097—5104 |
| [18] | Bao N., Shen L., Takata T., Domen K., Gupta A., Yanagisawa K., Grimes C. A., J. Phys. Chem. C, 2007, 111(47), 17527—17534 |
| [19] | Khan U. A., Liu J., Pan J., Ma H., Zuo S., Yu Y., Ahmad A., Li B., Mat. Sci. Semicond. Process., 2019, 83, 201—210 |
| [20] | Lin L., Luo Y., Tsai P., Wang J., Chen X.,TrAC-Trends Anal. Chem., 2019, 103, 87—101 |
| [21] | Rashid J., Saleem S., Awan S. U., Iqbal A., Kumar R., Barakat M. A., Arshad M., Zaheer M., Rafique M., Awad M., RSC Adv., 2019, 8(22), 11935—11945 |
| [22] | Bella M., Rivero C., Blayac S., Basti H., Record M. C., Boulet P., Mater. Res. Bull., 2017, 90, 188—194 |
| [23] | Cheng L., Xiang Q., Liao Y., Zhang H., Energy Environ.Sci., 2019, 11(6), 1362—1391 |
| [24] | Murillo Leo I., Soto E., Vaquero F., Mota N., Navarro R. M., Fierro J. L. G., Int. J. Hydrogen Energy, 2017, 42(19), 13691—13703 |
| [25] | Gao S., Zhang J., Li Y., Jiao S., Yuan J., Wang G., Li X., Wang J., Yu Q., Zhang X., Eur. J. Inorg.Chem., 2019, 2018(18), 1916—1920 |
| [26] | Giri A., Park G., Yang H., Pal M., Kwak J., Jeong U., Adv. Mater., 2019, 30(25), 1707577—1707595 |
| [27] | Xu X., Hu L., Gao N., Liu S., Wageh S., Al-Ghamdi A. A., Alshahrie A., Fang X., Adv. Funct. Mater., 2015, 25(3), 445—454 |
| [28] | Hernández-Guzmán F., Nicho-Díaz M.E., Medrano-Solís A., Altuzar-Coello P., Eur. Polym. J., 2017, 90, 407—417 |
| [29] | And N. H. T., Lamb R. N., J. Phys. Chem. B, 2002, 106(2), 352—355 |
| [30] | Pezeshkpour S., Salamatinia B., Amini H. B., Ceram. Int., 2019, 44(3), 3201—3210 |
| [31] | Fang X., Jiao L., Zhang R., Jiang H. L., ACS Appl. Mater. Interfaces, 2017, 9(28), 23852—23858 |
| [32] | Deng Y., Zhang Y., Peng L., Jing X., Chen H., Adv. Mater. Sci.Eng., 2016, 2016, 1—10 |
| [33] | Zhang K., Liu X., Appl. Surf. Sci., 2011, 257(24), 10379—10383 |
| [34] | Ma T., Zhou F., Zhang T. W., Yao H. B., Su T. Y., Yu Z. L., Li Y., Lu L. L., Yu S. H., Angew. Chem. Int. Ed. Engl., 2017, 56(39), 11836—11840 |
| [35] | Azqhandi M. H. A., Vasheghani F B., Rajabi F. H., Keramati M., Results Phys., 2017, 7, 1106—1114 |
| [36] | Li C., Wang H., Naghadeh S. B., Zhang J. Z., Fang P., Appl. Catal. B: Environ., 2019, 227, 229—239 |
| [37] | Xu X., Hu L., Gao N., Liu S., Wageh S., Al-Ghamdi A. A., Alshahrie A., Fang X., Adv. Funct. Mater., 2014, 25(3), 445—454 |
| [38] | Banerji S., Byrne R. E., Livingstone S. E., Transition Met. Chem., 1982, 7(1), 5—10 |
| [39] | Guo M., Wu Q., Yu M., Wang Y., Li M., Electrochim. Acta, 2017, 236, 280—287 |
| [40] | Li R., Yu L., Yan X., Tang Q., RSC Adv., 2015, 5(16), 11917—11924 |
| [41] | Zhou J., Tian G., Chen Y., Shi Y., Tian C., Pan K., Fu H., Sci. Rep.-UK, 2014, 4(2955), 4027—4034 |
| [42] | Reddy C. V., Shim J., Cho M., J. Phys. Chem. Solids, 2017, 103, 209—217 |
| [43] | González-Moya J. R., Garcia-Basabe Y., Rocco M. L., Pereira M. B., Princival J. L., Almeida L. C., Araújo C. M., David D. G., Da S. A., Machado G., Nanotechnology, 2016, 27(28), 285401—285414 |
| [44] | Tong X. L., Jiang D. S., Liu Z. M., Luo M. Z., Li Y., Lu P. X., Yang G., Long H., Thin Solid Films, 2008, 516(8), 2003—2008 |
| [45] | Rondiya S., Rokade A., Funde A., Kartha M., Pathan H., Jadkar S., Thin Solid Films, 2017, 631, 41—49 |
| [46] | Qian J., Zhao Z., Shen Z., Zhang G., Peng Z., Fu X., J. Mater. Res., 2015, 30(24), 3746—3756 |
| [47] | Ye M., Wen X., Zhang N., Guo W., Liu X. Y., Lin C., J. Mater. Chem. A, 2015, 3(18), 9595—9600 |
| [48] | Cheng F., Yin H., Xiang Q., Appl. Surf. Sci., 2017, 391, 432—439 |
| [49] | Vamvasakis I., Trapali A., Miao J., Liu B., Armatas G. S., Inorg. Chem. Front., 2016, 4(3), 433—441 |
| [50] | Zou S., Fu Z. H., Zeng M., Zhou J. B., Journal of Natural Science of Hunan Normal University, 2016, 39(5), 57—60 |
| (邹帅, 伏再辉, 曾明, 周建波. 湖南师范大学自然科学学报, 2016, 39(5), 57—60) | |
| [51] | Xin Y., Chen Q., Zhang G., J. Alloy. Compd., 2019, 751, 231—240 |
| [52] | Wang Y., Yang X., Ye T., Xu C., Xia F., Meng D., J. Electron. Mater., 2016, 46(3), 1598—1606 |
| [53] | Li Q., Wang F., Sun L., Jiang Z., Ye T., Chen M., Bai Q., Wang C., Han X., Nano-Micro Lett., 2017, 9(3), 131—139 |
| [54] | Gao X. M., Dai Y., Fei J., Zhang Y., Fu F., Chem. J. Chinese Universities, 2017, 38(7), 1249—1256 |
| (高晓明, 代源, 费娇, 张裕, 付峰. 高等学校化学学报, 2017, 38(7), 1249—1256) | |
| [55] | Li X., Xia T., Xu C., Murowchick J., Chen X., Catal. Today, 2014, 225, 64—73 |
| [56] | Chen X., Li H., Wu Y., Wu H., Wu L., Tan P., Pan J., Xiong X., J. Colloid Interface Sci., 2016, 476, 132—143 |
| [57] | Pelaez M., Falaras P., Likodimos V., O’Shea K., de la Cruz A. A., Dunlop P. S. M., Byrne J. A., Dionysiou D. D., J. Mol. Catal. A: Chem., 2016, 425, 183—189 |
| [58] | Zou C., Meng Z., Ji W., Liu S., Shen Z., Zhang Y., Jiang N., Chinese J.Catal., 2019, 39(6), 1051—1059 |
| [1] | 滕镇远, 张启涛, 苏陈良. 聚合物单原子光催化剂的载流子分离和表面反应机制[J]. 高等学校化学学报, 2022, 43(9): 20220325. |
| [2] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [3] | 林治, 彭志明, 贺韦清, 沈少华. 单原子与团簇光催化: 竞争与协同[J]. 高等学校化学学报, 2022, 43(9): 20220312. |
| [4] | 赵盈喆, 张建玲. 金属-有机框架基材料在二氧化碳光催化转化中的应用[J]. 高等学校化学学报, 2022, 43(7): 20220223. |
| [5] | 夏雾, 任颖异, 刘京, 王锋. 壳聚糖包裹CdSe量子点组装体的水相可见光催化CO2还原[J]. 高等学校化学学报, 2022, 43(7): 20220192. |
| [6] | 邱丽琪, 姚向阳, 何良年. 可见光驱动丰产金属卟啉类配合物催化的二氧化碳选择性还原反应[J]. 高等学校化学学报, 2022, 43(7): 20220064. |
| [7] | 龚妍熹, 王建兵, 柴歩瑜, 韩元春, 马云飞, 贾超敏. 钾掺杂g-C3N4薄膜光阳极的制备及光电催化氧化降解水中双氯芬酸钠性能[J]. 高等学校化学学报, 2022, 43(6): 20220005. |
| [8] | 王广琦, 毕艺洋, 王嘉博, 石洪飞, 刘群, 张钰. 非贵金属三元复合Ni(PO3)2-Ni2P/CdS NPs异质结的构建及可见光高效催化产氢性能[J]. 高等学校化学学报, 2022, 43(6): 20220050. |
| [9] | 宋颖颖, 黄琳, 李庆森, 陈立妙. CuO/BiVO4光催化剂的制备及光催化CO2还原性能[J]. 高等学校化学学报, 2022, 43(6): 20220126. |
| [10] | 陶雨, 欧鸿辉, 雷永鹏, 熊禹. 单原子催化剂在光催化二氧化碳还原中的研究进展[J]. 高等学校化学学报, 2022, 43(5): 20220143. |
| [11] | 冯丽, 邵兰兴, 李思骏, 全文选, 庄金亮. 超薄Sm-MOF纳米片的合成及可见光催化降解芥子气模拟剂性能[J]. 高等学校化学学报, 2022, 43(4): 20210867. |
| [12] | 孟祥钰, 詹琦, 武亚南, 马晓双, 姜靖逸, 孙岳明, 代云茜. 光热效应增强的Au/RGO/Na2Ti3O7光催化加氢性能[J]. 高等学校化学学报, 2022, 43(3): 20210655. |
| [13] | 郭彪, 赵晨灿, 刘芯辛, 于洲, 周丽景, 袁宏明, 赵震. 表面水热碳层对磁性NiFe2O4八面体光催化活性的影响[J]. 高等学校化学学报, 2022, 43(11): 20220472. |
| [14] | 邵文惠, 胡欣, 尚静, 林峰, 金黎明, 权春善, 张艳梅, 李军. 高效广谱复合光催化抗菌剂Ag-AgVO3/BiVO4的设计合成及抗菌机制[J]. 高等学校化学学报, 2022, 43(10): 20220132. |
| [15] | 姜珊, 申倩倩, 李琦, 贾虎生, 薛晋波. Pd增强缺陷态TiO2纳米管阵列的光催化产氢性能[J]. 高等学校化学学报, 2022, 43(10): 20220206. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||