

高等学校化学学报 ›› 2019, Vol. 40 ›› Issue (10): 2111.doi: 10.7503/cjcu20190108
方芳1,薛良敏1,丛婧1,田超1,王孝伟1,刘俊义1,2,张志丽1,*( )
)
收稿日期:2019-02-17
出版日期:2019-10-08
发布日期:2019-06-01
通讯作者:
张志丽
E-mail:Lilybmu@bjmu.edu.cn
基金资助:
FANG Fang1,XUE Liangmin1,CONG Jing1,TIAN Chao1,WANG Xiaowei1,LIU Junyi1,2,ZHANG Zhili1,*( )
)
Received:2019-02-17
Online:2019-10-08
Published:2019-06-01
Contact:
ZHANG Zhili
E-mail:Lilybmu@bjmu.edu.cn
Supported by:摘要:
以非经典叶酸拮抗剂2,4-二氨基-6-(4-甲基苯基)乙基吡啶并[3,2-d]嘧啶(wm-5b)及其侧链简化产物2,4-二氨基吡啶并[3,2-d]嘧啶为先导化合物, 选取具有抗肿瘤活性的基团, 通过微波法高效合成了2-位或4-位取代吡啶并嘧啶类非经典叶酸拮抗剂, 研究了2-位及4-位取代基对抗肿瘤活性的影响, 为非经典叶酸拮抗剂的设计合成提供了更多的理论依据. 目标化合物的结构均经核磁共振波谱(NMR)和质谱(MS)确证. 生物活性测定结果表明, 所有目标化合物均具有抗肿瘤活性, 其中, 6-(4-甲基苯基)乙基-4-氨基-2-(3-氯-4-氟苯基)氨基吡啶并[3,2-d]嘧啶(6b)对HL-60细胞的IC50=(4.09±0.48) μmol/L, 对A549细胞的IC50=(17.99±7.20) μmol/L, 而对HCT116细胞的IC50=(14.52±4.74) μmol/L; 部分目标化合物具有二氢叶酸还原酶抑制活性. 此外, 对部分目标化合物和先导物进行了二氢叶酸还原酶晶体结构的分子对接, 对活性结果和构效关系从分子水平上进行解释.
中图分类号:
TrendMD:
方芳,薛良敏,丛婧,田超,王孝伟,刘俊义,张志丽. 2-位或4-位取代吡啶并嘧啶类非经典叶酸拮抗剂的合成及抗肿瘤活性. 高等学校化学学报, 2019, 40(10): 2111.
FANG Fang,XUE Liangmin,CONG Jing,TIAN Chao,WANG Xiaowei,LIU Junyi,ZHANG Zhili. Synthesis and Anti-tumor Activity Evaluation of a Series of 2- or 4-Substituted Pyrido[3,2-d]pyrimidines as Nonclassical Antifolates †. Chem. J. Chinese Universities, 2019, 40(10): 2111.
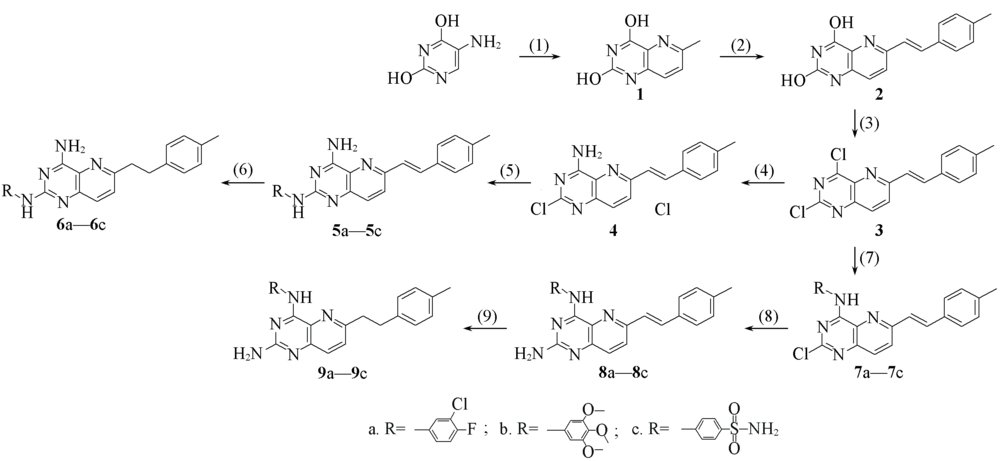
Fig.3 Scheme 1 Synthesis of 2- or 4-substituted 6-(4'-methylphenyl) ethylpyrido [3,2-d]pyrimidines Reagents and conditions: (1) CH3CH=CHCHO, 20%HCl; (2) 4-tolylaldehyde, PTSA, DMAC, 160 ℃, 36 h; (3) POCl3, NEt3; (4) NH3, CH3OH; (5) R1NH2, TFA, TFE, microwave, 140 ℃; (6) Pd/C, H2, CH3CH2OH; (7) R1NH2, CH3OH or CH3CH2OH; (8) NH3/CH3OH, 130 ℃, 130 h; (9) Pd/C, H2, CH3CH2OH.
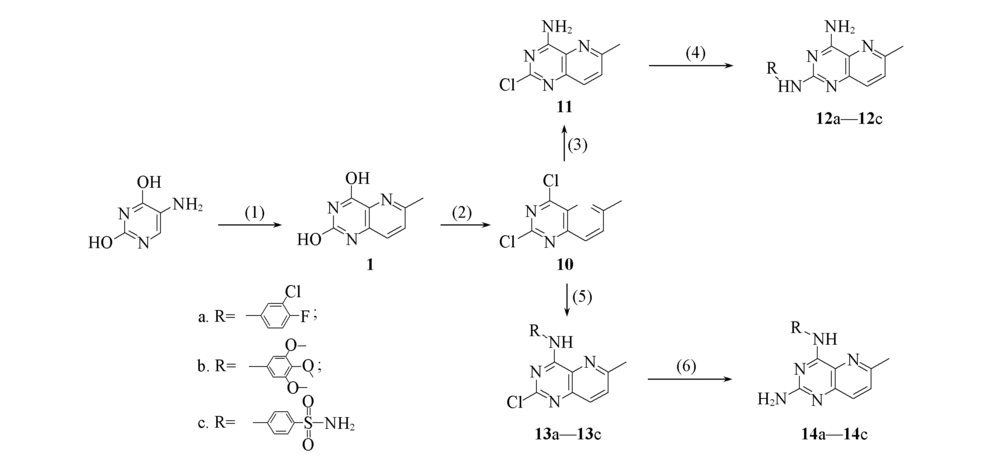
Fig.4 Scheme 2 Synthesis of 2- or 4-substituted 6-methylpyrido [3,2-d] pyrimidines Reagents and conditions: (1) CH3CH=CHCHO, 20%HCl; (2) POCl3, NEt3; (3) NH3, CH3OH; (4) R1NH2, TFA, TFE, microwave, 140 ℃; (5) R1NH2, CH3OH or CH3CH2OH; (6) NH3/CH3OH, 130 ℃, 130 h.
| Compd. | Name | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | (E)-2-Chloro-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-amine | 8.54(s, 1H), 8.44(s, 1H), 8.06(d,J=16.1 Hz, 1H), 7.98(s, 2H), 7.59(d, J=8.1 Hz, 2H), 7.37(d, J=16.1 Hz, 1H), 7.26(d, J=8.0 Hz, 2H), 2.34(s, 3H) | 163.60, 156.86, 154.69, 145.23, 138.97, 135.55, 135.27, 133.95, 130.36, 130.01, 128.58, 127.70, 126.08, 21.42 | ||||||||
| 5a | (E)-N2-(3-Chloro-4-fluorophenyl)-6-(4-methylstyryl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.39(s, 1H), 8.29(dd,J=6.9, 2.5 Hz, 1H), 7.88(m, J=10.0 Hz, 1H), 7.85(d, J=2.8 Hz, 1H), 7.83(s, 1H), 7.7(d, J=8.6 Hz, 2H),7.71(s, 1H), 7.57(d, J=7.9 Hz, 2H), 7.33(d, J=2.8 Hz, 1H), 7.31—7.27(m, 1H), 7.24(d, J=7.8 Hz,2H), 2.34(s, 3H) | 162.25, 156.93, 153.20, 150.90(d, J=21.1 Hz), 150.55, 145.33, 139.04, 138.25, 134.33, 133.68, 132.96, 129.92, 129.02, 127.69, 127.36, 126.80, 119.95, 119.34(d, J=17.1 Hz), 116.79(d, J=21.1 Hz), 21.37 | ||||||||
| 5b | (E)-6-(4-Methylstyryl)-N2-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.04(s, 1H), 7.85(d,J=16.3 Hz, 1H), 7.82(d, J=8.8 Hz, 1H), 7.76(s, 1H), 7.70(d, J=28.3 Hz, 2H), 7.57(d, J=8.0 Hz, 2H), 7.40(s, 2H), 7.31(d, J=16.2 Hz, 1H), 7.24(d, J=7.9 Hz, 2H), 3.79(s, 6H), 3.62(s, 3H), 2.34(s, 3H) | 162.09, 157.14, 153.00, 150.22, 138.28, 137.66, 134.38, 133.61, 132.72, 132.35, 129.95, 128.93, 127.63, 127.36, 126.92, 97.19, 60.53, 56.02, 21.33 | ||||||||
| 5c | (E)-4-((4-Amino-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-2-yl)amino)benzenesulfonamide | 9.61(s, 1H), 8.10(d,J=8.7 Hz, 2H),7.90(d, J=16.3 Hz, 2H), 7.84(d, J=10.1 Hz, 2H), 7.79(s, 1H), 7.71(d, J=8.8 Hz, 2H), 7.58(d, J=7.9 Hz, 2H), 7.33(d, J=16.2 Hz, 1H), 7.25(d, J=7.9 Hz, 2H), 7.17(s, 2H), 2.34(s, 3H) | 162.33, 156.93, 150.81, 144.82, 138.38, 135.99, 134.33, 133.84, 133.13, 129.95, 129.04, 127.76, 127.41, 126.80, 118.30, 70.34, 21.40 | ||||||||
| 7a | (E)-2-Chloro-N-(3-chloro-4-fluorophenyl)-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-amine | 10.45(s, 1H), 8.27(dd,J=6.8, 2.6 Hz, 1H), 8.17(d, J=16.2 Hz, 1H), 8.11(t, J=5.9 Hz, 2H), 8.01(m, J=9.0, 4.2, 2.7 Hz, 1H), 7.64(d, J=8.0 Hz, 2H), 7.55(t, J= 9.1 Hz, 1H), 7.47(d, J=16.2 Hz, 1H), 7.29(d, J=7.9 Hz, 2H), 2.36(s, 3H) | 158.66, 155.79, 155.61(s, J=227 Hz), 145.33, 139.22, 136.50, 135.75, 135.57, 133.84, 130.49, 130.07, 128.93, 127.84, 126.08, 124.70, 123.57, 123.50, 117.39(d, J=25 Hz), 21.45 | ||||||||
| Compd. | Name | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ | ||||||||
| 7b | (E)-2-Chloro-6-(4-methylstyryl)-N-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidin-4-amine | 10.18(s, 1H), 8.11(d, J=16.5 Hz, 2H), 8.07(s, 1H), 7.63(d, J=8.0 Hz, 2H), 7.49(s, 2H), 7.45(d, J=16.3 Hz, 1H), 7.28(d, J=8.0 Hz, 2H), 3.85(s, 6H), 3.71(s, 3H), 2.35(s, 3H) | 158.37, 155.99, 155.43, 153.08, 145.19, 139.20, 136.26, 135.66, 134.97, 134.22, 133.83, 130.55, 130.03, 128.45, 127.80, 126.22, 100.67, 60.64, 56.36, 21.45 | ||||||||
| 7c | (E)-4-((2-Chloro-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 10.55(s, 1H), 8.22—8.13(m, 5H), 7.91(d,J=8.8 Hz, 2H), 7.65(d, J=8.0 Hz, 2H), 7.50(d, J=16.2 Hz, 1H), 7.37(s, 2H), 7.30(d, J=8.0 Hz, 2H), 2.36(s, 3H) | 158.73, 155.75, 145.50, 141.26, 140.07, 139.32, 136.61, 135.83, 133.83, 130.55, 130.08, 128.95, 127.86, 126.86, 126.12, 122.55, 21.45 | ||||||||
| 8a | (E)-N4-(3-Chloro-4-fluorophenyl)-6-(4-methylstyryl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.79(s, 1H), 8.46(dd,J=6.8, 2.7 Hz, 1H), 8.21(m, J=9.1, 4.3, 2.7 Hz, 1H), 7.91(d, J=16.3 Hz, 1H), 7.88(d, J=8.8 Hz, 1H), 7.68(d, J=8.7 Hz, 1H), 7.62(d, J=8.1 Hz, 2H), 7.45(t, J=9.1 Hz, 1H), 7.38(d, J=16.3 Hz, 1H), 7.28(d, J=8.0 Hz, 2H), 6.73(s, 2H), 2.37(s, 3H) | 160.82, 157.73, 154.72, 152.31(d, J=241 Hz), 149.47, 146.57, 138.53, 136.92, 134.35, 132.94, 132.82, 129.93, 127.70, 127.35, 126.90, 122.85, 121.80, 119.47(d, J=18.2 Hz), 116.94(d, J=21.7 Hz), 21.35 | ||||||||
| 8b | (E)-6-(4-Methylstyryl)-N4-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.49(s, 1H), 7.88(d,J=8.8 Hz, 1H), 7.83(d, J=16.4 Hz, 1H), 7.64(d, J=8.7 Hz, 1H), 7.60(d, J=8.0 Hz, 2H), 7.56(s, 2H), 7.36(d, J=16.4 Hz, 1H), 7.25(d, J=8.0 Hz, 2H), 6.63(s, 2H), 3.86(s, 6H), 3.67(s, 3H), 2.34(s, 3H) | 160.23, 157.61, 153.14, 149.30, 146.62, 138.28, 135.56, 134.35, 133.76, 133.01, 132.56, 129.93, 127.86, 127.35, 127.17, 99.21, 60.59, 56.48, 21.39 | ||||||||
| 8c | (E)-4-((2-Amino-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 9.88(s, 1H), 8.39(d,J=8.4 Hz, 2H), 7.91(d, J=11.3 Hz, 1H), 7.86(d, J=18.3 Hz, 2H), 7.81(s, 1H), 7.68(d, J=9.0 Hz, 1H), 7.61(d, J=8.0 Hz, 2H), 7.38(d, J=16.3 Hz, 1H), 7.31(s, 2H), 7.26(d, J=7.6 Hz), 6.78(s, 2H), 2.35(s, 3H) | 160.14, 157.69, 149.57, 146.95, 142.66, 138.36, 138.26, 134.34, 133.21, 132.91, 129.95, 127.73, 127.68, 127.40, 126.95, 126.79, 120.69, 21.40 | ||||||||
| 13a | 2-Chloro-N-(3-chloro-4-fluorophenyl)-6-methylpyrido[3,2-d]pyrimidin-4-amine | 10.46(s, 1H), 8.29—8.23(m, 1H), 8.08(d,J=8.6 Hz, 1H), 7.99(m, J=9.0, 4.3, 2.7 Hz, 1H), 7.85(d, J=8.6 Hz, 1H), 7.52(t, J=9.1 Hz, 1H), 2.78(s, 3H) | 159.30, 158.56, 155.61(d,J=243 Hz), 153.15, 144.92, 135.63(d, J=3.0 Hz), 135.38, 130.55, 129.79, 124.39, 123.20, 119.43(d, J=18.5 Hz), 117.14(d, J=21.7 Hz), 24.93 | ||||||||
| 13b | 2-Chloro-6-methyl-N-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidin-4-amine | 10.15(s, 1H, NH-4), 8.06(d,J=8.5 Hz, 1H), 7.83(d, J=8.6 Hz, 1H), 7.52(s, 2H), 3.84(s, 6H), 3.70(s, 3H), 2.77(s, 3H) | 159.07, 158.23, 155.80, 152.98, 144.68, 135.37, 134.71, 134.33, 130.36, 129.88, 100.25, 60.55, 56.49, 24.83 | ||||||||
| 13c | 4-((2-Chloro-6-methylpyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 10.51(s, 1H), 8.13(d,J=8.8 Hz, 2H), 8.10—8.04(m, 1H), 7.87(d, J=8.8 Hz, 2H), 7.83(m, J=5.4, 3.1 Hz, 1H), 7.33(s, 2H), 2.77(s, 3H) | 159.49, 158.69, 155.67, 145.06, 141.29, 139.92, 135.45, 130.68, 129.85, 126.80, 122.35, 24.89 | ||||||||
| 6a | N2-(3-Chloro-4-fluorophenyl)-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.32(s, 1H), 8.28(d,J=6.7 Hz, 1H), 7.84(d, J=9.1 Hz, 1H), 7.70(d, J=8.5 Hz, 1H), 7.58(s, 2H), 7.52(s, J=8.7 Hz, 1H), 7.29(t, J=9.1 Hz, 1H), 7.14(d, J=7.6 Hz, 2H), 7.07(d, J=7.6 Hz, 2H), 3.11(d, J=6.6 Hz, 2H), 3.07(d, J=5.7 Hz, 2H), 2.25(s, 3H) | 162.14, 156.81, 156.30, 153.20(d, J=238 Hz), 150.82, 144.85, 139.23, 138.90, 135.10, 133.63, 129.27, 128.77, 128.73, 128.42, 119.78, 119.00(d, J=20.9, 12.1 Hz), 116.77(d, J=21.4 Hz), 34.57, 21.29, 14.23 | ||||||||
| 6b | 6-(4-Methylphenethyl)-N2-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 8.96(s, 1H), 7.68(d,J=8.5 Hz, 1H), 7.48(s, 1H), 7.45(d, J=11.3 Hz, 2H), 7.42(s, 2H), 7.12(d, J=7.9 Hz, 2H), 7.05(d, J=7.9 Hz, 2H), 3.79(s, 6H), 3.62(s, 3H), 3.09(t, 2H), 3.05(t, 2H), 2.23(s, 3H) | 161.96, 157.13, 155.84, 152.97, 145.03, 138.85, 137.94, 135.07, 133.66, 132.14, 129.24, 128.69, 128.61, 128.35, 97.02, 60.54, 56.10, 34.65, 21.01, 18.99 | ||||||||
| Compd. | Name | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ | ||||||||
| 6c | 4-((4-Amino-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidin-2-yl)amino)benzenesulfonamide | 9.57(s, 1H), 8.12(d,J=8.8 Hz, 2H), 7.78(d, J=8.6 Hz, 1H), 7.71(d, J=8.8 Hz, 2H), 7.64(d, J=10.2 Hz, 2H), 7.58(d, J=8.6 Hz, 1H), 7.18(d, J=9.7 Hz, 4H), 7.10(d, J=7.9 Hz, 2H), 3.15(t, 2H), 3.10(t, 2H), 2.28(s, 3H) | 162.19, 156.80, 156.57, 144.96, 144.88, 138.89, 135.78, 135.12, 133.79, 129.29, 128.83, 128.75, 128.41, 126.78, 118.08, 34.52, 31.75, 21.09 | ||||||||
| 9a | N4-(3-Chloro-4-fluorophenyl)-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.62(s, 1H), 8.41(dd,J=6.8, 2.5 Hz, 1H), 8.18—8.09(m, 1H), 7.61(d, J=8.6 Hz, 1H), 7.53(d, J=8.6 Hz, 1H), 7.43(t, J=9.1 Hz, 1H), 7.18(d, J=7.8 Hz, 2H), 7.10(d, J=7.8 Hz, 2H), 6.62(s, 2H), 3.23—3.14(t, 2H), 3.13—3.05(t, 2H), 2.27(s, 3H) | 159.96, 157.63, 155.15, 154.63(d, J=241 Hz), 152.22, 146.03, 138.90, 136.93, 135.11, 132.96, 129.27, 128.73, 127.11, 122.64, 121.57, 119.45(d, J=18.2 Hz), 116.91(d, J=21.6 Hz), 36.64, 30.39, 22.03 | ||||||||
| 9b | 6-(4-Methylphenethyl)-N4-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.34(s, 1H), 7.59(d,J=8.5 Hz, 1H), 7.52(d, 1H), 7.50(s, 2H), 7.17(d, J=6.9 Hz, 2H), 7.09(d, J=7.4 Hz, 2H), 6.53(s, 2H), 3.86(s, 6H), 3.67(s, 3H), 3.17(t, 2H), 3.09(t, 2H), 2.26(s, 3H) | 160.07, 157.54, 154.94, 153.15, 145.91, 138.84, 135.56, 135.15, 133.67, 133.00, 129.28, 128.76, 127.30, 98.98, 60.59, 56.47, 34.86, 30.48, 21.10 | ||||||||
| 9c | 4-((2-Amino-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 9.71(s, 1H), 8.31(d,J=8.5 Hz,2H), 7.80(d, J=8.6 Hz, 2H), 7.62(d, J=8.6 Hz, 1H), 7.54(d, J=8.7 Hz, 1H), 7.31(s, 2H), 7.17(d, J=7.6 Hz, 2H), 7.09(d, J=7.5 Hz, 2H), 6.69(s, 2H), 3.17(t, 2H), 3.09(t, 2H), 2.25(s, 3H) | 159.79, 157.48, 155.27, 145.56, 142.28, 138.72, 137.99, 135.10, 132.98, 129.29, 128.95, 128.77, 127.08, 126.79, 120.32, 34.55, 29.48, 21.12 | ||||||||
| 12a | N2-(3-Chloro-4-fluorophenyl)-6-methylpyrido[3,2-d]pyrimidine-2,4-diamine | 9.34(s, 1H), 8.32(dd,J=6.9, 2.5 Hz, 1H), 7.91—7.83(m, 1H), 7.75(d, J=8.6 Hz, 1H), 7.57(d, J=8.6 Hz, 1H), 7.54(s, 2H), 7.32(t, J=9.1 Hz, 1H), 2.62(s, 3H) | 162.06, 156.74, 153.43, 153.31(d, J=238 Hz), 144.66, 139.27, 133.77, 129.20, 128.40, 119.74(d, J=29 Hz), 118.99(d, J=23.6 Hz), 116.88, 116.67, 24.44 | ||||||||
| 12b | 6-Methyl-N2-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 8.96(s, 1H), 7.74(d,J=8.5 Hz, 1H), 7.56(d, J=8.6 Hz, 1H), 7.45(s, 2H), 7.44(s, 2H), 3.83(s, 6H), 3.66(s, 3H), 2.62(s, 3H) | 161.87, 157.06, 152.97, 144.88, 137.95, 133.81, 132.14, 129.06, 128.31, 97.03, 60.58, 56.13, 24.43 | ||||||||
| 12c | 4-((4-Amino-6-methylpyrido[3,2-d]pyri-midin-2-yl)amino)benzenesulfonamide | 10.95(s, 1H), 9.27(d,J=97.5 Hz, 2H), 7.95(d, J=8.6 Hz, 1H), 7.87(m, J=20.7, 8.9 Hz, 4H), 7.77(d, J=8.6 Hz, 1H), 7.38(s, 2H), 2.65(s, 3H) | 162.93, 156.57, 153.04, 150.33, 140.90, 139.87, 131.09, 127.17, 126.99, 126.40, 121.56, 24.07 | ||||||||
| 14a | N4-(3-Chloro-4-fluorophenyl)-6-methylpyrido[3,2-d]pyrimidine-2,4-diamine | 9.68(s, 1H), 8.43(dd,J=6.8, 2.3 Hz, 1H), 8.18—8.04(m, 1H), 7.62(d, J=8.6 Hz, 1H), 7.53(d, J=8.6 Hz, 1H), 7.41(t, J=9.1 Hz, 1H), 6.58(s, 2H), 2.64(s, 3H) | 159.95, 157.58, 154.55, 152.14(d, J=236 Hz), 145.97, 137.09, 133.11, 129.26, 127.15, 122.54, 121.47, 119.40(d, J=18.2 Hz), 116.87(d, J=21.5 Hz), 24.29 | ||||||||
| 14b | 6-Methyl-N4-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.37(s, 1H), 7.60(d,J=8.6 Hz, 1H), 7.53(s, 2H), 7.51(d, J=8.6 Hz, 1H), 6.51(s, 2H), 3.85(s, 6H), 3.66(s, 3H), 2.63(s, 3H) | 159.99, 157.44, 153.12, 151.85, 145.66, 135.67, 133.53, 133.08, 129.09, 127.29, 98.78, 60.55, 56.40, 24.72 | ||||||||
| 14c | 4-((2-Amino-6-methylpyrido[3,2-d]pyri-midin-4-yl)amino)benzenesulfonamide | 9.78(s, 1H, NH-4), 8.34(d, J=8.8 Hz, 2H), 7.81(d, J=8.8 Hz, 2H), 7.65(d, J=8.6 Hz, 1H), 7.56(d, J=8.6 Hz, 1H), 7.32(s, 2H), 6.65(s, 2H), 2.65(s, 3H) | 159.90, 157.44, 152.29, 146.07, 142.63, 137.96, 133.10, 129.39, 127.08, 126.76, 120.46, 24.32 | ||||||||
Table 1 1H NMR and 13C NMR data of compounds
| Compd. | Name | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | (E)-2-Chloro-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-amine | 8.54(s, 1H), 8.44(s, 1H), 8.06(d,J=16.1 Hz, 1H), 7.98(s, 2H), 7.59(d, J=8.1 Hz, 2H), 7.37(d, J=16.1 Hz, 1H), 7.26(d, J=8.0 Hz, 2H), 2.34(s, 3H) | 163.60, 156.86, 154.69, 145.23, 138.97, 135.55, 135.27, 133.95, 130.36, 130.01, 128.58, 127.70, 126.08, 21.42 | ||||||||
| 5a | (E)-N2-(3-Chloro-4-fluorophenyl)-6-(4-methylstyryl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.39(s, 1H), 8.29(dd,J=6.9, 2.5 Hz, 1H), 7.88(m, J=10.0 Hz, 1H), 7.85(d, J=2.8 Hz, 1H), 7.83(s, 1H), 7.7(d, J=8.6 Hz, 2H),7.71(s, 1H), 7.57(d, J=7.9 Hz, 2H), 7.33(d, J=2.8 Hz, 1H), 7.31—7.27(m, 1H), 7.24(d, J=7.8 Hz,2H), 2.34(s, 3H) | 162.25, 156.93, 153.20, 150.90(d, J=21.1 Hz), 150.55, 145.33, 139.04, 138.25, 134.33, 133.68, 132.96, 129.92, 129.02, 127.69, 127.36, 126.80, 119.95, 119.34(d, J=17.1 Hz), 116.79(d, J=21.1 Hz), 21.37 | ||||||||
| 5b | (E)-6-(4-Methylstyryl)-N2-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.04(s, 1H), 7.85(d,J=16.3 Hz, 1H), 7.82(d, J=8.8 Hz, 1H), 7.76(s, 1H), 7.70(d, J=28.3 Hz, 2H), 7.57(d, J=8.0 Hz, 2H), 7.40(s, 2H), 7.31(d, J=16.2 Hz, 1H), 7.24(d, J=7.9 Hz, 2H), 3.79(s, 6H), 3.62(s, 3H), 2.34(s, 3H) | 162.09, 157.14, 153.00, 150.22, 138.28, 137.66, 134.38, 133.61, 132.72, 132.35, 129.95, 128.93, 127.63, 127.36, 126.92, 97.19, 60.53, 56.02, 21.33 | ||||||||
| 5c | (E)-4-((4-Amino-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-2-yl)amino)benzenesulfonamide | 9.61(s, 1H), 8.10(d,J=8.7 Hz, 2H),7.90(d, J=16.3 Hz, 2H), 7.84(d, J=10.1 Hz, 2H), 7.79(s, 1H), 7.71(d, J=8.8 Hz, 2H), 7.58(d, J=7.9 Hz, 2H), 7.33(d, J=16.2 Hz, 1H), 7.25(d, J=7.9 Hz, 2H), 7.17(s, 2H), 2.34(s, 3H) | 162.33, 156.93, 150.81, 144.82, 138.38, 135.99, 134.33, 133.84, 133.13, 129.95, 129.04, 127.76, 127.41, 126.80, 118.30, 70.34, 21.40 | ||||||||
| 7a | (E)-2-Chloro-N-(3-chloro-4-fluorophenyl)-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-amine | 10.45(s, 1H), 8.27(dd,J=6.8, 2.6 Hz, 1H), 8.17(d, J=16.2 Hz, 1H), 8.11(t, J=5.9 Hz, 2H), 8.01(m, J=9.0, 4.2, 2.7 Hz, 1H), 7.64(d, J=8.0 Hz, 2H), 7.55(t, J= 9.1 Hz, 1H), 7.47(d, J=16.2 Hz, 1H), 7.29(d, J=7.9 Hz, 2H), 2.36(s, 3H) | 158.66, 155.79, 155.61(s, J=227 Hz), 145.33, 139.22, 136.50, 135.75, 135.57, 133.84, 130.49, 130.07, 128.93, 127.84, 126.08, 124.70, 123.57, 123.50, 117.39(d, J=25 Hz), 21.45 | ||||||||
| Compd. | Name | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ | ||||||||
| 7b | (E)-2-Chloro-6-(4-methylstyryl)-N-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidin-4-amine | 10.18(s, 1H), 8.11(d, J=16.5 Hz, 2H), 8.07(s, 1H), 7.63(d, J=8.0 Hz, 2H), 7.49(s, 2H), 7.45(d, J=16.3 Hz, 1H), 7.28(d, J=8.0 Hz, 2H), 3.85(s, 6H), 3.71(s, 3H), 2.35(s, 3H) | 158.37, 155.99, 155.43, 153.08, 145.19, 139.20, 136.26, 135.66, 134.97, 134.22, 133.83, 130.55, 130.03, 128.45, 127.80, 126.22, 100.67, 60.64, 56.36, 21.45 | ||||||||
| 7c | (E)-4-((2-Chloro-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 10.55(s, 1H), 8.22—8.13(m, 5H), 7.91(d,J=8.8 Hz, 2H), 7.65(d, J=8.0 Hz, 2H), 7.50(d, J=16.2 Hz, 1H), 7.37(s, 2H), 7.30(d, J=8.0 Hz, 2H), 2.36(s, 3H) | 158.73, 155.75, 145.50, 141.26, 140.07, 139.32, 136.61, 135.83, 133.83, 130.55, 130.08, 128.95, 127.86, 126.86, 126.12, 122.55, 21.45 | ||||||||
| 8a | (E)-N4-(3-Chloro-4-fluorophenyl)-6-(4-methylstyryl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.79(s, 1H), 8.46(dd,J=6.8, 2.7 Hz, 1H), 8.21(m, J=9.1, 4.3, 2.7 Hz, 1H), 7.91(d, J=16.3 Hz, 1H), 7.88(d, J=8.8 Hz, 1H), 7.68(d, J=8.7 Hz, 1H), 7.62(d, J=8.1 Hz, 2H), 7.45(t, J=9.1 Hz, 1H), 7.38(d, J=16.3 Hz, 1H), 7.28(d, J=8.0 Hz, 2H), 6.73(s, 2H), 2.37(s, 3H) | 160.82, 157.73, 154.72, 152.31(d, J=241 Hz), 149.47, 146.57, 138.53, 136.92, 134.35, 132.94, 132.82, 129.93, 127.70, 127.35, 126.90, 122.85, 121.80, 119.47(d, J=18.2 Hz), 116.94(d, J=21.7 Hz), 21.35 | ||||||||
| 8b | (E)-6-(4-Methylstyryl)-N4-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.49(s, 1H), 7.88(d,J=8.8 Hz, 1H), 7.83(d, J=16.4 Hz, 1H), 7.64(d, J=8.7 Hz, 1H), 7.60(d, J=8.0 Hz, 2H), 7.56(s, 2H), 7.36(d, J=16.4 Hz, 1H), 7.25(d, J=8.0 Hz, 2H), 6.63(s, 2H), 3.86(s, 6H), 3.67(s, 3H), 2.34(s, 3H) | 160.23, 157.61, 153.14, 149.30, 146.62, 138.28, 135.56, 134.35, 133.76, 133.01, 132.56, 129.93, 127.86, 127.35, 127.17, 99.21, 60.59, 56.48, 21.39 | ||||||||
| 8c | (E)-4-((2-Amino-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 9.88(s, 1H), 8.39(d,J=8.4 Hz, 2H), 7.91(d, J=11.3 Hz, 1H), 7.86(d, J=18.3 Hz, 2H), 7.81(s, 1H), 7.68(d, J=9.0 Hz, 1H), 7.61(d, J=8.0 Hz, 2H), 7.38(d, J=16.3 Hz, 1H), 7.31(s, 2H), 7.26(d, J=7.6 Hz), 6.78(s, 2H), 2.35(s, 3H) | 160.14, 157.69, 149.57, 146.95, 142.66, 138.36, 138.26, 134.34, 133.21, 132.91, 129.95, 127.73, 127.68, 127.40, 126.95, 126.79, 120.69, 21.40 | ||||||||
| 13a | 2-Chloro-N-(3-chloro-4-fluorophenyl)-6-methylpyrido[3,2-d]pyrimidin-4-amine | 10.46(s, 1H), 8.29—8.23(m, 1H), 8.08(d,J=8.6 Hz, 1H), 7.99(m, J=9.0, 4.3, 2.7 Hz, 1H), 7.85(d, J=8.6 Hz, 1H), 7.52(t, J=9.1 Hz, 1H), 2.78(s, 3H) | 159.30, 158.56, 155.61(d,J=243 Hz), 153.15, 144.92, 135.63(d, J=3.0 Hz), 135.38, 130.55, 129.79, 124.39, 123.20, 119.43(d, J=18.5 Hz), 117.14(d, J=21.7 Hz), 24.93 | ||||||||
| 13b | 2-Chloro-6-methyl-N-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidin-4-amine | 10.15(s, 1H, NH-4), 8.06(d,J=8.5 Hz, 1H), 7.83(d, J=8.6 Hz, 1H), 7.52(s, 2H), 3.84(s, 6H), 3.70(s, 3H), 2.77(s, 3H) | 159.07, 158.23, 155.80, 152.98, 144.68, 135.37, 134.71, 134.33, 130.36, 129.88, 100.25, 60.55, 56.49, 24.83 | ||||||||
| 13c | 4-((2-Chloro-6-methylpyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 10.51(s, 1H), 8.13(d,J=8.8 Hz, 2H), 8.10—8.04(m, 1H), 7.87(d, J=8.8 Hz, 2H), 7.83(m, J=5.4, 3.1 Hz, 1H), 7.33(s, 2H), 2.77(s, 3H) | 159.49, 158.69, 155.67, 145.06, 141.29, 139.92, 135.45, 130.68, 129.85, 126.80, 122.35, 24.89 | ||||||||
| 6a | N2-(3-Chloro-4-fluorophenyl)-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.32(s, 1H), 8.28(d,J=6.7 Hz, 1H), 7.84(d, J=9.1 Hz, 1H), 7.70(d, J=8.5 Hz, 1H), 7.58(s, 2H), 7.52(s, J=8.7 Hz, 1H), 7.29(t, J=9.1 Hz, 1H), 7.14(d, J=7.6 Hz, 2H), 7.07(d, J=7.6 Hz, 2H), 3.11(d, J=6.6 Hz, 2H), 3.07(d, J=5.7 Hz, 2H), 2.25(s, 3H) | 162.14, 156.81, 156.30, 153.20(d, J=238 Hz), 150.82, 144.85, 139.23, 138.90, 135.10, 133.63, 129.27, 128.77, 128.73, 128.42, 119.78, 119.00(d, J=20.9, 12.1 Hz), 116.77(d, J=21.4 Hz), 34.57, 21.29, 14.23 | ||||||||
| 6b | 6-(4-Methylphenethyl)-N2-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 8.96(s, 1H), 7.68(d,J=8.5 Hz, 1H), 7.48(s, 1H), 7.45(d, J=11.3 Hz, 2H), 7.42(s, 2H), 7.12(d, J=7.9 Hz, 2H), 7.05(d, J=7.9 Hz, 2H), 3.79(s, 6H), 3.62(s, 3H), 3.09(t, 2H), 3.05(t, 2H), 2.23(s, 3H) | 161.96, 157.13, 155.84, 152.97, 145.03, 138.85, 137.94, 135.07, 133.66, 132.14, 129.24, 128.69, 128.61, 128.35, 97.02, 60.54, 56.10, 34.65, 21.01, 18.99 | ||||||||
| Compd. | Name | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ | ||||||||
| 6c | 4-((4-Amino-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidin-2-yl)amino)benzenesulfonamide | 9.57(s, 1H), 8.12(d,J=8.8 Hz, 2H), 7.78(d, J=8.6 Hz, 1H), 7.71(d, J=8.8 Hz, 2H), 7.64(d, J=10.2 Hz, 2H), 7.58(d, J=8.6 Hz, 1H), 7.18(d, J=9.7 Hz, 4H), 7.10(d, J=7.9 Hz, 2H), 3.15(t, 2H), 3.10(t, 2H), 2.28(s, 3H) | 162.19, 156.80, 156.57, 144.96, 144.88, 138.89, 135.78, 135.12, 133.79, 129.29, 128.83, 128.75, 128.41, 126.78, 118.08, 34.52, 31.75, 21.09 | ||||||||
| 9a | N4-(3-Chloro-4-fluorophenyl)-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.62(s, 1H), 8.41(dd,J=6.8, 2.5 Hz, 1H), 8.18—8.09(m, 1H), 7.61(d, J=8.6 Hz, 1H), 7.53(d, J=8.6 Hz, 1H), 7.43(t, J=9.1 Hz, 1H), 7.18(d, J=7.8 Hz, 2H), 7.10(d, J=7.8 Hz, 2H), 6.62(s, 2H), 3.23—3.14(t, 2H), 3.13—3.05(t, 2H), 2.27(s, 3H) | 159.96, 157.63, 155.15, 154.63(d, J=241 Hz), 152.22, 146.03, 138.90, 136.93, 135.11, 132.96, 129.27, 128.73, 127.11, 122.64, 121.57, 119.45(d, J=18.2 Hz), 116.91(d, J=21.6 Hz), 36.64, 30.39, 22.03 | ||||||||
| 9b | 6-(4-Methylphenethyl)-N4-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.34(s, 1H), 7.59(d,J=8.5 Hz, 1H), 7.52(d, 1H), 7.50(s, 2H), 7.17(d, J=6.9 Hz, 2H), 7.09(d, J=7.4 Hz, 2H), 6.53(s, 2H), 3.86(s, 6H), 3.67(s, 3H), 3.17(t, 2H), 3.09(t, 2H), 2.26(s, 3H) | 160.07, 157.54, 154.94, 153.15, 145.91, 138.84, 135.56, 135.15, 133.67, 133.00, 129.28, 128.76, 127.30, 98.98, 60.59, 56.47, 34.86, 30.48, 21.10 | ||||||||
| 9c | 4-((2-Amino-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 9.71(s, 1H), 8.31(d,J=8.5 Hz,2H), 7.80(d, J=8.6 Hz, 2H), 7.62(d, J=8.6 Hz, 1H), 7.54(d, J=8.7 Hz, 1H), 7.31(s, 2H), 7.17(d, J=7.6 Hz, 2H), 7.09(d, J=7.5 Hz, 2H), 6.69(s, 2H), 3.17(t, 2H), 3.09(t, 2H), 2.25(s, 3H) | 159.79, 157.48, 155.27, 145.56, 142.28, 138.72, 137.99, 135.10, 132.98, 129.29, 128.95, 128.77, 127.08, 126.79, 120.32, 34.55, 29.48, 21.12 | ||||||||
| 12a | N2-(3-Chloro-4-fluorophenyl)-6-methylpyrido[3,2-d]pyrimidine-2,4-diamine | 9.34(s, 1H), 8.32(dd,J=6.9, 2.5 Hz, 1H), 7.91—7.83(m, 1H), 7.75(d, J=8.6 Hz, 1H), 7.57(d, J=8.6 Hz, 1H), 7.54(s, 2H), 7.32(t, J=9.1 Hz, 1H), 2.62(s, 3H) | 162.06, 156.74, 153.43, 153.31(d, J=238 Hz), 144.66, 139.27, 133.77, 129.20, 128.40, 119.74(d, J=29 Hz), 118.99(d, J=23.6 Hz), 116.88, 116.67, 24.44 | ||||||||
| 12b | 6-Methyl-N2-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 8.96(s, 1H), 7.74(d,J=8.5 Hz, 1H), 7.56(d, J=8.6 Hz, 1H), 7.45(s, 2H), 7.44(s, 2H), 3.83(s, 6H), 3.66(s, 3H), 2.62(s, 3H) | 161.87, 157.06, 152.97, 144.88, 137.95, 133.81, 132.14, 129.06, 128.31, 97.03, 60.58, 56.13, 24.43 | ||||||||
| 12c | 4-((4-Amino-6-methylpyrido[3,2-d]pyri-midin-2-yl)amino)benzenesulfonamide | 10.95(s, 1H), 9.27(d,J=97.5 Hz, 2H), 7.95(d, J=8.6 Hz, 1H), 7.87(m, J=20.7, 8.9 Hz, 4H), 7.77(d, J=8.6 Hz, 1H), 7.38(s, 2H), 2.65(s, 3H) | 162.93, 156.57, 153.04, 150.33, 140.90, 139.87, 131.09, 127.17, 126.99, 126.40, 121.56, 24.07 | ||||||||
| 14a | N4-(3-Chloro-4-fluorophenyl)-6-methylpyrido[3,2-d]pyrimidine-2,4-diamine | 9.68(s, 1H), 8.43(dd,J=6.8, 2.3 Hz, 1H), 8.18—8.04(m, 1H), 7.62(d, J=8.6 Hz, 1H), 7.53(d, J=8.6 Hz, 1H), 7.41(t, J=9.1 Hz, 1H), 6.58(s, 2H), 2.64(s, 3H) | 159.95, 157.58, 154.55, 152.14(d, J=236 Hz), 145.97, 137.09, 133.11, 129.26, 127.15, 122.54, 121.47, 119.40(d, J=18.2 Hz), 116.87(d, J=21.5 Hz), 24.29 | ||||||||
| 14b | 6-Methyl-N4-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.37(s, 1H), 7.60(d,J=8.6 Hz, 1H), 7.53(s, 2H), 7.51(d, J=8.6 Hz, 1H), 6.51(s, 2H), 3.85(s, 6H), 3.66(s, 3H), 2.63(s, 3H) | 159.99, 157.44, 153.12, 151.85, 145.66, 135.67, 133.53, 133.08, 129.09, 127.29, 98.78, 60.55, 56.40, 24.72 | ||||||||
| 14c | 4-((2-Amino-6-methylpyrido[3,2-d]pyri-midin-4-yl)amino)benzenesulfonamide | 9.78(s, 1H, NH-4), 8.34(d, J=8.8 Hz, 2H), 7.81(d, J=8.8 Hz, 2H), 7.65(d, J=8.6 Hz, 1H), 7.56(d, J=8.6 Hz, 1H), 7.32(s, 2H), 6.65(s, 2H), 2.65(s, 3H) | 159.90, 157.44, 152.29, 146.07, 142.63, 137.96, 133.10, 129.39, 127.08, 126.76, 120.46, 24.32 | ||||||||
| Compd. | m. p./℃ | MS, m/z[M+H]+ | Compd. | m. p./℃ | MS, m/z[M+H]+ |
|---|---|---|---|---|---|
| 6a | 139—140 | 408.13 | 12a | 172—173 | 304.07 |
| 6b | 129—130 | 446.21 | 12b | 171—172 | 342.15 |
| 6c | 233—234 | 435.15 | 12c | >300 | 331.09 |
| 9a | 183—184 | 408.13 | 14a | 221 | 323.20 |
| 9b | 165—166 | 446.21 | 14b | 164 | 361.10 |
| 9c | 266—267 | 435.15 | 14c | 290—291 | 350.02 |
Table 2 Melt point and MS data for compounds 6a—6c, 9a—9c, 12a—12c and 14a—14c
| Compd. | m. p./℃ | MS, m/z[M+H]+ | Compd. | m. p./℃ | MS, m/z[M+H]+ |
|---|---|---|---|---|---|
| 6a | 139—140 | 408.13 | 12a | 172—173 | 304.07 |
| 6b | 129—130 | 446.21 | 12b | 171—172 | 342.15 |
| 6c | 233—234 | 435.15 | 12c | >300 | 331.09 |
| 9a | 183—184 | 408.13 | 14a | 221 | 323.20 |
| 9b | 165—166 | 446.21 | 14b | 164 | 361.10 |
| 9c | 266—267 | 435.15 | 14c | 290—291 | 350.02 |
| Compd. | IC50/(μmol·L-1) | Compd. | IC50/(μmol·L-1) | ||||
|---|---|---|---|---|---|---|---|
| HL-60 | A549 | HCT116 | HL-60 | A549 | HCT116 | ||
| 6a | 12.46±1.97 | 6.72±3.42 | 23.11±2.96 | 12b | 30.75±2.17 | 34.91±18.30 | 35.36±0.08 |
| 6b | 4.09±0.48 | 17.99±7.20 | 14.52±4.74 | 12c | 39.29±5.45 | 62.51±10.23 | >100 |
| 6c | 6.57±0.17 | 22.21±8.99 | >100 | 14a | 15.43±582 | 30.91±0.28 | 16.51±11.23 |
| 9a | 18.39±10.54 | ND | ND | 14b | 6.20±0.60 | 18.86±4.48 | 16.67±7.10 |
| 9b | 6.74±1.44 | 16.57±1.64 | 13.70±1.02 | 14c | 65.35±12.89 | 41.82±7.01 | 39.99±6.67 |
| 9c | 5.28±1.70 | 20.02±2.40 | 22.28±0.01 | wm-5b | 0.07±0.05 | 3.25±1.20 | 4.68±3.93 |
| 12a | 20.35±2.35 | 24.40±4.88 | 32.58±3.43 | MTX | 0.023±0.001 | 0.041±0.02 | 0.281±4.56 |
Table 3 IC50 values of target compounds against tumor cell lines*
| Compd. | IC50/(μmol·L-1) | Compd. | IC50/(μmol·L-1) | ||||
|---|---|---|---|---|---|---|---|
| HL-60 | A549 | HCT116 | HL-60 | A549 | HCT116 | ||
| 6a | 12.46±1.97 | 6.72±3.42 | 23.11±2.96 | 12b | 30.75±2.17 | 34.91±18.30 | 35.36±0.08 |
| 6b | 4.09±0.48 | 17.99±7.20 | 14.52±4.74 | 12c | 39.29±5.45 | 62.51±10.23 | >100 |
| 6c | 6.57±0.17 | 22.21±8.99 | >100 | 14a | 15.43±582 | 30.91±0.28 | 16.51±11.23 |
| 9a | 18.39±10.54 | ND | ND | 14b | 6.20±0.60 | 18.86±4.48 | 16.67±7.10 |
| 9b | 6.74±1.44 | 16.57±1.64 | 13.70±1.02 | 14c | 65.35±12.89 | 41.82±7.01 | 39.99±6.67 |
| 9c | 5.28±1.70 | 20.02±2.40 | 22.28±0.01 | wm-5b | 0.07±0.05 | 3.25±1.20 | 4.68±3.93 |
| 12a | 20.35±2.35 | 24.40±4.88 | 32.58±3.43 | MTX | 0.023±0.001 | 0.041±0.02 | 0.281±4.56 |
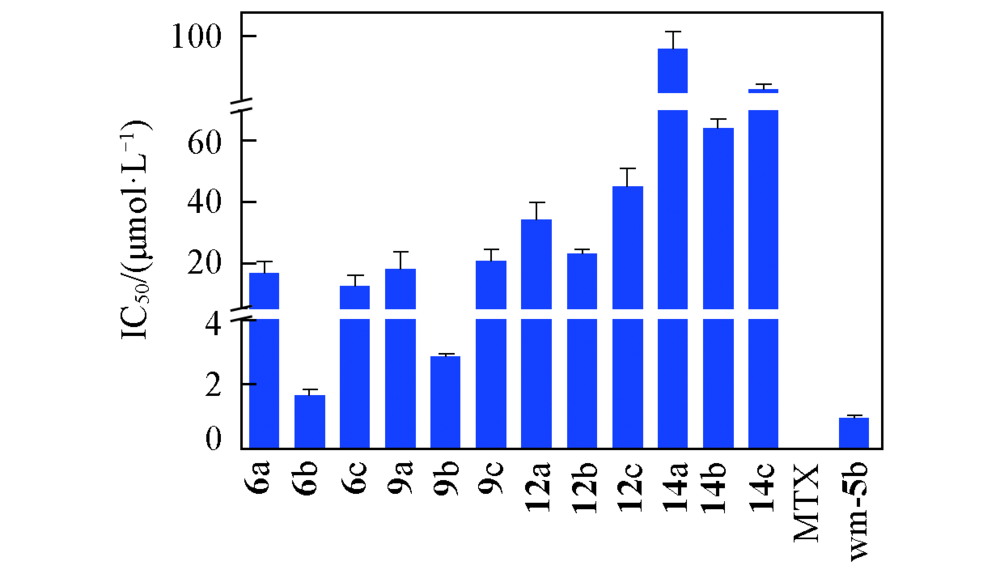
Fig.1 IC50 values of target compounds against DHFR Results are presented as mean values±standard errors from three replicates; MTX showed rhDHFR inhibitory potency with IC50 value of (0.0094±0.0002) μmol/L; calculated by three replicates.
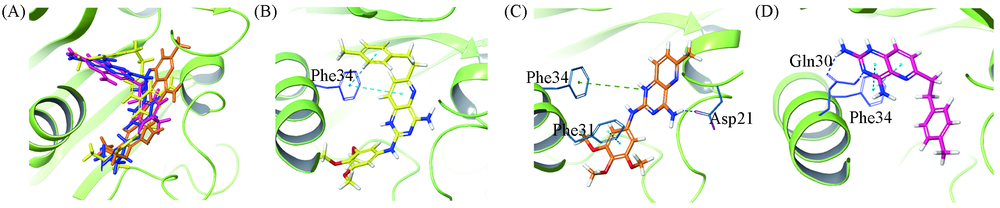
Fig.2 Results of docking of different compouds (A) Docking of the overlay of the original ligand and compounds wm-5b, 6b and 12b in rhDHFR; (B) docking of compound 6b; (C) docking of compound 12b; (D) docking of compound wm-5b. MTX: blue; compound 6b: yellow; compound 12b: orange and compound wm-5b: red(PDB code 4QJC).
| [1] | Filiz E. O. B., Hande S., Hulya A. , Pteridines, 2015,13(3), 85— 92 |
| [2] | Claudia M. H., Elisa T., Marilu F., Serena V. , Expert Opinion on Drug Metabolism & Toxicology, 2017,26(3), 245— 257 |
| [3] | Xia Q., Li R. L. , World Pharmacy, 1997,18(3), 135— 140 |
| ( 夏青, 李仁利 . 世界临床药物, 1997,18(3), 135— 140 | |
| [4] | Brigle K. E., Spinella M. J., Sierra E. E., Goldman I. D. , J. Biol. Chem., 1995,270(39), 22974— 22979 |
| [5] | Robert M., Godefridus J. P., David G. P., Yehuda G. A., Stavit D. Ietje K., Paul N., Marlene A. B., Andre R., Jan H. S., Herbert M. P., Gerrit J. , Biochemical Pharmacology, 2002,63(2), 105— 115 |
| [6] | Raz S., Stark M., Assaraf Y. G. , Drug Resistance Updates, 2016,28, 43— 64 |
| [7] | Lilah R., Ilan I., Yotam K., David G. P., Gerrit J., Yehuda G. A. , Biochem. J., 2002,367(3), 741— 750 |
| [8] | Assaraf Y. G., Schimke R. T. , Proc. Nat. Acad. Sci.USA, 1987,84(20), 7154— 7158 |
| [9] | Taylor I. W., Slowiaczek P., Friedlander M. L., Tattersall M. H. , Cancer Research, 1985,45(3), 978— 982 |
| [10] | Webber S., Bartlett C. A., Boritzki T. J., Hillard J. A., Howland E. F. Johnston A. L., Kosa M., Margosiak S. A., Morse C. A., Shetty B. V. , Cancer Chemotherapy and Pharmacology, 1996,37(6), 509— 517 |
| [11] | Carbain B., Coxon C. R. , Lebraud H., Elliott K. J., Matheson C. J., Meschini E., Roberts A. R., Turner D. M., Wong C., Cano C., Griffin R. J., Hardcastle I. R., Golding B. T. , Chem. Eur. J., 2014,20, 2311— 2317 |
| [12] | Andrew J. B., Gibson K. H., Walter G., Andrew A. G., Jeffrey J. B., Mark P. H., James R. W., Susan E. A., Brenda J. C., Lynn S., Lianne H., Laura R. , Bio. & Med. Chem. Lett., 2001,14(11), 1911— 1914 |
| [13] | Wissner A., Mansour T. S. , Arch. Pharm. Chem. Life Sci., 2008,341, 465— 477 |
| [14] | Kong X. L. , J. Int. Oncol., 1992,19(3) , 144— 146 |
| ( 孔祥林 . 国际肿瘤学杂志, 1992,19(3), 144— 146) | |
| [15] | Ernst S., Stephane B., Riazul A., Mathew P. , J. Med. Chem., 2013,56, 3768— 3782 |
| [16] | Liu X. R., Shi S. H., Lam F., Pepper C., Fischer P. M., Wang S. D. , Int. J. Cancer, 2012,130, 1216— 1226 |
| [17] | Lam F., Abbas A. Y., Shao H., Teo T., Adams J. Li P., Bradshaw T. D., Fischer P. M., Walsby E., Pepper C., Chen Y., Ding J., Wang S. D. , Oncotarget, 2014,17(5), 7691— 7704 |
| [18] | Tao Z., Cheng H. B., Zhou J. P., Zhang H. B. , Progress in Pharmaceutical Sciences, 2013,37(6), 241— 248 |
| ( 陶卓, 程海博, 周金培, 张惠斌 . 药学进展, 2013,37(6), 241— 248) | |
| [19] | Wang M., Yang J. J., Yuan M. M., Xue L. M., Li H., Tian C., Wang X. W., Liu J. Y., Zhang L. L. , Eur. J. Med., 2017,128, 88— 97 |
| [20] | El-Subbagh H. I., Hassan G. S., El-Messery S. M., Al-Rashood S. T., Al-Omary F. A. M., Abulfadl Y. S., Shabayek M. , Eur. J. Med. Chem., 2014,74, 234— 245 |
| [21] | He X., Gong H., Li L., Guo Y. D. , Guangdong Chemical Industry, 2015,45(2), 70— 71 |
| ( 何兴, 龚浩, 李玲, 郭义东 . 广东化工, 2015,45(2), 70— 71) | |
| [22] | Carbain B., Coxoh C. R., Lebraud H., Elliott K. J., Matheson C. J., Meschini E., Roberts A. R., Turner D. M., Wong C., Cano C., Griffin R. J., Hardcastle I. R., Golding B. T. , Chem. Eur. J., 2014,20(8), 2311— 2317 |
| [1] | 肖艳华, 张广杰, 宗良, 刘国宏, 任丽君, 董俊兴. 开口箭化学成分及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(9): 1897. |
| [2] | 吕明君, 李雯, 杨新颖, 方浩. N9位芳基取代嘌呤-8-酮类衍生物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(2): 254. |
| [3] | 张培全,杨倩倩,龙惠丹,陈鑫. 金诺芬衍生物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(10): 2097. |
| [4] | 刘莉, 马洋洋, 王宽, 贾云静, 李婉, 朱华结. β-咔啉衍生物的抗肿瘤及抗菌活性[J]. 高等学校化学学报, 2018, 39(4): 674. |
| [5] | 王磊, 郑国钧, 季奇, 陈博, 巩龙龙, 高聪敏, 杜镇建, 张兴民. PI3K/mTOR抑制剂的合成及生物活性[J]. 高等学校化学学报, 2017, 38(9): 1590. |
| [6] | 白信法, 马宣, 解晓霞, 邵明莎, 郭宁宁, 严宁, 姚雷. 微管菌素类似物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2017, 38(1): 47. |
| [7] | 史蕾, 杨文聪, 曾淑莹, 莫婷婷, 张召, 曹曼丽, 刘海洋. 咔咯钴(Ⅲ)配合物与DNA的相互作用及抗肿瘤活性[J]. 高等学校化学学报, 2016, 37(6): 1059. |
| [8] | 郭亮, 曹日晖, 范文玺, 甘紫云, 马芹. 双-β-咔啉衍生物的设计、合成及抗肿瘤活性[J]. 高等学校化学学报, 2016, 37(6): 1093. |
| [9] | 张洁, 周昌健, 谢建伟, 代斌. 大黄酸-缬氨酸加合物的合成及初步抗肿瘤活性[J]. 高等学校化学学报, 2016, 37(12): 2159. |
| [10] | 周皓, 段志刚, 赵爽, 宝梅英, 李志伟, 裴亚中. 嘧啶联苯类化合物的设计、 合成及抗肿瘤活性[J]. 高等学校化学学报, 2015, 36(9): 1694. |
| [11] | 王刚, 韩雷强, 方浩. 苯基哌嗪衍生物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2015, 36(12): 2435. |
| [12] | 郭华, 杨承玲, 王蔚, 赖全勇, 袁直. 基于海藻酸钠衍生物的肝靶向纳米前药的构建及抗肿瘤活性研究[J]. 高等学校化学学报, 2014, 35(8): 1835. |
| [13] | 童春义, 唐凤霞, 刘斌, 廖红东, 刘选明. 氟尿嘧啶-壳寡糖/硒纳米微球的制备及抑制肿瘤细胞生长活性[J]. 高等学校化学学报, 2014, 35(7): 1603. |
| [14] | 李小六, 马东来, 杨海龙, 谭官海, 杜会茹, 王克让, 张平竹, 陈华. 吖啶-多胺类缀合物的合成、 抗肿瘤活性及与DNA键合作用[J]. 高等学校化学学报, 2014, 35(6): 1181. |
| [15] | 王军华, 王泉德, 顿艳艳, 方浩. 嘌呤磺胺衍生物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2014, 35(6): 1189. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||