

高等学校化学学报 ›› 2015, Vol. 36 ›› Issue (9): 1694.doi: 10.7503/cjcu20150404
周皓, 段志刚, 赵爽, 宝梅英, 李志伟( ), 裴亚中(
), 裴亚中( )
)
收稿日期:2015-05-20
出版日期:2015-09-10
发布日期:2015-08-21
作者简介:联系人简介: 裴亚中, 男, 博士, 教授, 博士生导师, 主要从事多样化合成与创新药物研究. E-mail:基金资助:
ZHOU Hao, DUAN Zhigang, ZHAO Shuang, BAO Meiying, LI Zhiwei*( ), PEI Yazhong*(
), PEI Yazhong*( )
)
Received:2015-05-20
Online:2015-09-10
Published:2015-08-21
Contact:
LI Zhiwei,PEI Yazhong
E-mail:zwl.jida@gmail.com;peiyz@jlu.edu.cn
Supported by:摘要:
设计合成了一系列以嘧啶联苯为中心结构的潜在激酶变构抑制剂. 以2,4-二氯-5-甲基嘧啶为起始原料, 通过Suzuki偶联一锅法合成了中心药效团嘧啶联苯, 通过X射线单晶衍射分析确认结构, 并在此基础上衍生化合成了19个化合物. 采用噻唑蓝(MTT)法对所得化合物用人乳腺癌细胞(MCF-7)进行体外抗肿瘤活性初步筛选, 并找到2个具有潜在应用价值的化合物8c(IC50=0.224 μmol/L)和9c(IC50=0.113 μmol/L).
中图分类号:
TrendMD:
周皓, 段志刚, 赵爽, 宝梅英, 李志伟, 裴亚中. 嘧啶联苯类化合物的设计、 合成及抗肿瘤活性. 高等学校化学学报, 2015, 36(9): 1694.
ZHOU Hao, DUAN Zhigang, ZHAO Shuang, BAO Meiying, LI Zhiwei, PEI Yazhong. Design and Synthesis of Phenylpyrimidine and Their Anticancer Activity†. Chem. J. Chinese Universities, 2015, 36(9): 1694.
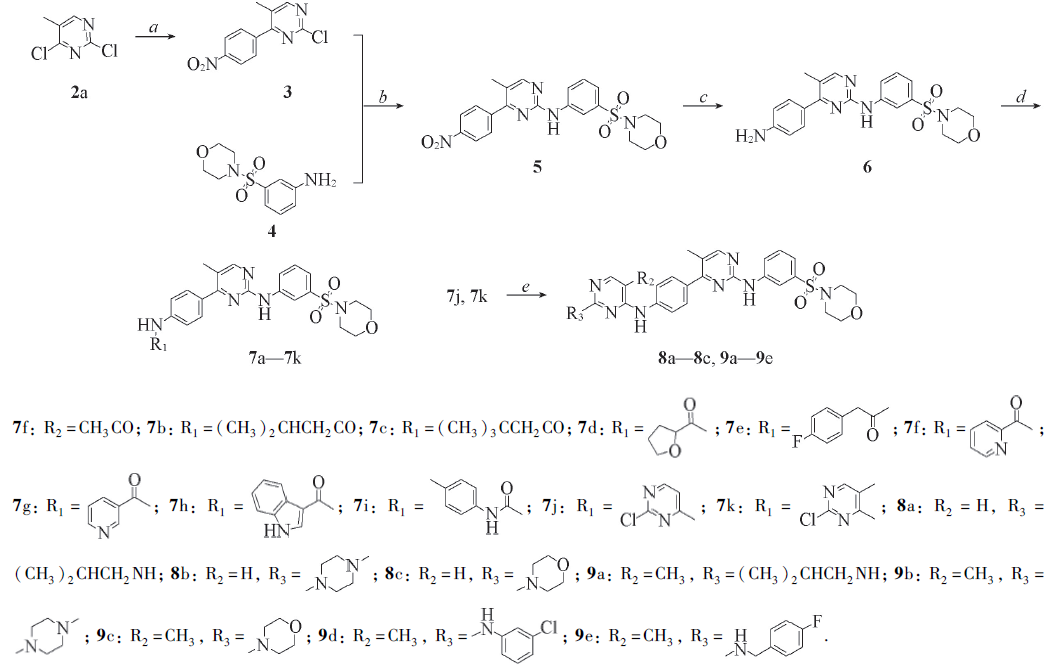
Scheme 1 General syntheses of target compoundsReagents and conditions: a. bis(pinacolato)diboron(2b), 1-bromo-4-nitrobenzene(2c), Pd(PPh3)4, KOAc, Na2CO3, N2, Dioxane/H2O, 107 ℃; b. p-TsOH, Dioxane, reflux; c. Fe, NH4Cl, EtOH/H2O, reflux; d. (1) RCOOH, DMAP, EDCI, DCM, or (2) p-tolyl isocyanate, TEA, DCM, or (3) R1Cl, TEA, n-BuOH; e. R3H, p-TsOH, dioxane, 100 ℃.
| Compd. | m. p./℃ | MS(calcd.), m/z[M+H+] | Compd. | m. p./℃ | MS(calcd.), m/z[M+H+] |
|---|---|---|---|---|---|
| 3 | 118—120 | 250.0(249.0) | 7i | 187—189 | 559.8(558.2) |
| 4 | 124—126 | 243.3(242.0) | 7j | 113—115 | 538.1(537.1) |
| 5 | 196—198 | 456.4(455.1) | 7k | 115—117 | 552.1(551.2) |
| 6 | 196—198 | 426.4(425.2) | 8a | 199—201 | 575.4(574.3) |
| 7a | 173—175 | 468.2(467.2) | 8b | 126—128 | 602.3(601.2) |
| 7b | 194—195 | 510.6(509.2) | 8c | 127—129 | 589.3(588.2) |
| 7c | 227—229 | 524.4(523.2) | 9a | 127—129 | 589.4(588.3) |
| 7d | 215—217 | 524.6(523.2) | 9b | 127—129 | 616.3(615.3) |
| 7e | 158—197 | 562.6(561.2) | 9c | 210—212 | 603.4(602.2) |
| 7f | 189—191 | 531.6(530.2) | 9d | 225—227 | 643.5(642.2) |
| 7g | 186—188 | 531.5(530.2) | 9e | 140—142 | 641.5(640.2) |
| 7h | 177—179 | 569.2(568.2) |
Table 1 Melting points and MS data of compounds 3—6, 7a—7k, 8a—8c and 9a—9e
| Compd. | m. p./℃ | MS(calcd.), m/z[M+H+] | Compd. | m. p./℃ | MS(calcd.), m/z[M+H+] |
|---|---|---|---|---|---|
| 3 | 118—120 | 250.0(249.0) | 7i | 187—189 | 559.8(558.2) |
| 4 | 124—126 | 243.3(242.0) | 7j | 113—115 | 538.1(537.1) |
| 5 | 196—198 | 456.4(455.1) | 7k | 115—117 | 552.1(551.2) |
| 6 | 196—198 | 426.4(425.2) | 8a | 199—201 | 575.4(574.3) |
| 7a | 173—175 | 468.2(467.2) | 8b | 126—128 | 602.3(601.2) |
| 7b | 194—195 | 510.6(509.2) | 8c | 127—129 | 589.3(588.2) |
| 7c | 227—229 | 524.4(523.2) | 9a | 127—129 | 589.4(588.3) |
| 7d | 215—217 | 524.6(523.2) | 9b | 127—129 | 616.3(615.3) |
| 7e | 158—197 | 562.6(561.2) | 9c | 210—212 | 603.4(602.2) |
| 7f | 189—191 | 531.6(530.2) | 9d | 225—227 | 643.5(642.2) |
| 7g | 186—188 | 531.5(530.2) | 9e | 140—142 | 641.5(640.2) |
| 7h | 177—179 | 569.2(568.2) |
| Compd. | 1H NMR(300 MHz), δa | 13C NMR(75 MHz), δb |
|---|---|---|
| 3 | 8.60(d, J=0.7 Hz, 1H), 8.44—8.34(m, 2H), 7.89—7.79(m, 2H), 2.41(d, J=0.7 Hz, 3H) | 165.6, 162.1, 142.6, 130.2, 129.0, 127.1, 125.6, 123.9, 16.5 |
| 5 | 10.12(s,1H), 8.57(s,1H), 8.44(t, J=1.9 Hz,1H), 8.41—8.32(m, 2H), 8.02—7.91(m, 3H), 7.55(t, J=8.0 Hz, 1H), 7.29—7.23(m, 1H), 3.62—3.54(m, 4H), 2.87—2.79(m, 4H), 2.26(s, 3H) | 162.3, 160.5, 158.0, 147.6, 144.0, 141.4, 134.6, 130.0, 129.4, 123.2, 122.5, 119.6, 119.1, 116.6, 65.1, 45.8, 15.4 |
| 6 | 9.83(s, 1H), 8.61(s, 1H), 8.34(s, 1H), 7.94—7.80(m, 1H), 7.64—7.46(m, 3H), 7.32—7.13(m, 1H), 6.66(d, J=8.6 Hz, 2H), 5.58(s, 2H), 3.66—3.53(m, 4H), 2.90—2.82(m, 4H), 2.30(s, 3H) | 164.0, 159.5, 158.0, 150.1, 142.0, 134.6, 130.2, 129.2, 124.7, 122.2, 119.1, 117.8, 116.1, 112.8, 65.1, 45.8, 16.8 |
| 7a | 10.18(s, 1H), 10.00(s, 1H), 8.60(t, J=1.8 Hz, 1H), 8.48(s, 1H), 7.95—7.88(m, 1H), 7.75(s, 4H), 7.56(t, J=8.0 Hz, 1H), 7.30—7.24(m, 1H), 3.65—3.57(m, 4H), 2.91—2.84(m, 4H), 2.31(s, 3H), 2.12(s, 3H) | 168.7, 163.8, 160.1, 158.2, 141.9, 140.4, 134.7, 132.4, 129.6, 129.5, 122.6, 119.5, 118.7, 118.3, 116.5, 65.3, 46.0, 24.1, 16.3 |
| 7b | 10.08(s, 1H), 9.98(s, 1H), 8.57(s, 1H), 8.45(s, 1H), 7.91—7.85(m, 1H), 7.79—7.67(m, 4H), 7.54(t, J=8.0 Hz, 1H), 7.28—7.19(m, 1H), 3.62—3.52(m, 4H), 2.90—2.78(m, 4H), 2.28(s, 3H), 2.23(d, J=7.0 Hz, 2H), 2.18—2.02(m, 1H), 0.96(d, J=6.5 Hz, 6H) | 171.0, 163.8, 160.1, 158.2, 141.9, 140.4, 134.7, 132.4, 129.5, 129.4, 122.5, 119.5, 118.7, 118.4, 116.5, 65.3, 45.9, 45.7, 25.6, 22.3, 16.2 |
| 7c | 10.01(s, 1H), 9.97(s, 1H), 8.59—8.54(m, 1H), 8.45(s, 1H), 7.92—7.85(m, 1H), 7.79—7.66(m, 4H), 7.54(t, J=8.0 Hz, 1H), 7.27—7.21(m, 1H), 3.61—3.54(m, 4H), 2.88—2.79(m, 4H), 2.29(s, 3H), 2.24(s, 2H), 1.05(s, 9H) | 170.3, 163.7, 160.1, 158.1, 141.9, 140.2, 134.6, 132.4, 129.5, 129.4, 122.5, 119.5, 118.7, 118.5, 116.5, 65.2, 49.6, 45.9, 30.9, 29.6, 16.2 |
| 7d | 9.98(s, 1H), 9.90(s, 1H), 8.59—8.54(m, 1H), 8.46(s, 1H), 7.93—7.82(m, 3H), 7.72(d, J=8.7 Hz, 2H), 7.54(t, J=8.0 Hz, 1H), 7.27—7.21(m, 1H), 4.43(dd, J=8.1, 5.5 Hz, 1H), 4.08—3.95(m, 1H), 3.92—3.79(m, 1H), 3.65—3.50(m, 4H), 2.91—2.78(m, 4H), 2.28(s, 3H), 2.27—2.16(m, 1H), 2.09—1.95(m, 1H), 1.95—1.81(m, 2H) | 171.8, 163.8, 160.1, 158.1, 141.8, 139.4, 134.7, 133.0, 129.4, 129.3, 122.5, 119.5, 119.2, 118.7, 116.4, 77.9, 68.8, 65.2, 45.9, 29.9, 25.1, 16.2 |
| 7e | 10.39(s, 1H), 9.98(s, 1H), 8.57(s, 1H), 8.45(s, 1H), 7.92—7.85(m, 1H), 7.78—7.68(m, 4H), 7.53(t, J=8.0 Hz, 1H), 7.43—7.34(m, 2H), 7.27—7.10(m, 3H), 3.69(s, 2H), 3.61—3.52(m, 4H), 2.88—2.78(m, 4H), 2.28(s, 3H) | 169.3, 163.7, 161.1(d, J=242.1 Hz), 160.1, 158.1, 141.9, 140.2, 134.7, 132.7, 132.0(d, J=3.1 Hz), 131.0(d, J=8.0 Hz), 129.6, 129.4, 122.5, 119.5, 118.7, 118.5, 116.5, 115.0(d, J=21.2 Hz), 65.2, 45.9, 42.4, 16.2 |
| 7f | 10.88(s, 1H), 10.01(s, 1H), 8.82—8.76(m, 1H), 8.59—8.56(m, 1H), 8.49(s, 1H), 8.25—8.18(m, 1H), 8.15—8.06(m, 3H), 7.97—7.87(m, 1H), 7.79(d, J=8.7 Hz, 2H), 7.75—7.69(m, 1H), 7.55(t, J=8.0 Hz, 1H), 7.30—7.22(m, 1H), 3.66—3.54(m, 4H), 2.91—2.83(m, 4H), 2.32(s, 3H) | 169.1, 168.0, 165.4, 163.4, 155.0, 153.7, 147.1, 144.6, 143.4, 140.0, 138.6, 136.7, 134.7, 133.9, 132.3, 127.7, 124.9, 124.8, 124.1, 121.7, 70.5, 51.2, 21.4 |
| 7g | 10.68(s, 1H), 10.03(s, 1H), 9.27—9.08(m, 1H), 8.88—8.76(m, 1H), 8.62(s, 1H), 8.51(s, 1H), 8.40—8.30(m, 1H), 8.00—7.90(m, 3H), 7.82(d, J=8.7 Hz, 2H), 7.66—7.51(m,2H), 7.34—7.22(m,1H), 3.73—3.52(m, 4H), 2.99—2.79(m, 4H), 2.34(s, 3H) | 164.3, 163.7, 160.2, 158.2, 152.2, 148.8, 141.9, 139.9, 135.6, 134.6, 133.4, 130.5, 129.5, 129.4, 123.5, 122.6, 119.7, 119.5, 118.8, 116.5, 65.3, 46.0, 16.2 |
| 7h | 11.79(s, 1H), 9.98(s, 1H), 9.94(s, 1H), 8.67—8.59(m, 1H), 8.47(s, 1H), 8.36(d, J=3.0 Hz, 1H), 8.27—8.19(m, 1H), 8.00—7.87(m, 3H), 7.78(d, J=8.7 Hz, 2H), 7.61—7.46(m, 2H), 7.29—7.13(m, 3H), 3.64—3.52(m, 4H), 2.93—2.79(m, 4H), 2.33(s, 3H) | 163.9, 163.4, 160.1, 158.2, 141.9, 141.0, 136.2, 134.7, 131.9, 129.5, 129.0, 128.7, 126.4, 122.5, 122.2, 121.1, 120.8, 119.5, 118.9, 118.7, 116.5, 112.0, 110.4, 65.3, 45.9, 16.3 |
| 7i | 9.96(s, 1H), 8.91(s, 1H), 8.64(s, 1H), 8.60—8.53(m,1H), 8.45(s, 1H), 7.94—7.87(m, 1H), 7.72(d, J=8.7 Hz, 2H), 7.64—7.49(m, 3H), 7.37(d, J=8.4 Hz, 2H), 7.26—7.21(m, 1H), 7.10(d, J=8.4 Hz, 2H), 3.62—3.56(m, 4H), 2.89—2.82(m, 4H), 2.30(s, 3H), 2.25(s, 3H) | 163.9, 160.1, 158.2, 152.5, 141.9, 141.1, 137.0, 134.7, 131.1, 130.9, 129.7, 129.4, 129.2, 122.5, 119.5, 118.7, 118.4, 117.4, 116.5, 65.3, 46.0, 20.4, 16.4 |
| 7j | 8.54—8.48(m, 1H), 8.36(s, 1H), 8.18(d, J=5.8 Hz, 1H), 7.83(s, 1H), 7.73(d, J=8.5 Hz, 2H), 7.68—7.60(m, 1H), 7.57—7.43(m,4H), 7.42—7.33(m, 1H), 6.71(d, J=5.8 Hz, 1H), 3.73—3.65(m, 4H), 3.06—2.95(m, 4H), 2.34(s, 3H) | 163.6, 161.2, 160.1, 159.3, 158.1, 157.3, 141.8, 139.9, 134.6, 132.6, 129.7, 129.4, 122.5, 119.5, 119.2, 118.7, 116.4, 106.4, 65.2, 45.9, 16.2 |
| 7k | 10.00(s, 1H), 9.03(s, 1H), 8.60(s, 1H), 8.48(s, 1H), 8.12(s, 1H), 7.96—7.73(m, 5H), 7.54(t, J=8.0 Hz, 1H), 7.25(d, 1H), 3.67—3.53(m, 4H), 2.94—2.76(m, 4H), 2.32(s, 3H), 2.23(s, 3H) | 163.7, 160.1, 160.0, 158.2, 156.7, 156.6, 141.9, 139.8, 134.7, 133.0, 129.4, 129.2, 122.5, 121.1, 119.5, 118.7, 116.4, 114.9, 65.3, 45.9, 16.3, 13.5 |
| 8a | 9.94(s, 1H), 9.42(s, 1H), 8.54(s, 1H), 8.42(s, 1H), 7.95—7.83(m, 4H), 7.69(d, J=8.7 Hz, 2H), 7.51(t, J=8.0 Hz, 1H), 7.27—7.20(m, 1H), 6.95(s, 1H), 6.02(d, J=5.7 Hz, 1H), 3.60—3.50(m, 4H), 3.08(t, J=6.4 Hz, 2H), 2.89—2.75(m, 4H), 2.29(s, 3H), 1.95—1.80(m, 1H), 0.89(d, J=6.7 Hz, 6H) | 163.9, 162.0, 160.4, 160.0, 158.2, 156.5, 156.3, 141.9, 134.7, 130.6, 129.4, 126.4, 122.5, 119.4, 118.6, 118.3, 117.3, 116.5, 65.3, 48.6, 45.9, 27.8, 20.3, 16.4 |
| 8b | 9.99(s, 1H), 9.55(s, 1H), 8.75(s, 1H), 8.45(s, 1H), 7.99(d, J=5.6 Hz, 1H), 7.88—7.74(m, 5H), 7.54(t, J=8.0 Hz, 1H), 7.30—7.21(m, 1H), 6.12(d, J=5.6 Hz, 1H), 3.79—3.68(m, 4H), 3.62—3.51(m, 4H), 2.91—2.80(m, 4H), 2.46—2.38(m, 4H), 2.34(s, 3H), 2.23(s, 3H) | 163.5, 161.1, 160.1, 160.0, 158.1, 156.2, 141.9, 141.5, 134.8, 130.7, 129.6, 129.2, 122.4, 119.3, 118.4, 118.3, 116.3, 97.0, 65.2, 54.4, 45.8, 45.7, 43.4, 16.4 |
| 8c | 9.99(s, 1H), 9.58(s, 1H), 8.77(s, 1H), 8.45(s, 1H), 8.01(d, J=5.6 Hz, 1H), 7.90—7.75(m, 5H), 7.53(t, J=8.0 Hz, 1H), 7.30—7.21(m, 1H), 6.15(d, J=5.6 Hz, 1H), 3.70(s, 8H), 3.59—3.50(m, 4H), 2.91—2.81(m, 4H), 2.34(s, 3H) | 163.4, 161.3, 160.3, 160.2, 158.1, 156.3, 141.9, 141.5, 134.6, 130.8, 129.7, 129.4, 122.5, 119.4, 118.5, 118.3, 116.3, 97.6, 66.1, 65.2, 45.9, 44.2, 16.5 |
| 9a | 9.97(s, 1H), 8.59(t, J=1.7 Hz, 1H), 8.45(s, 1H), 8.26(s, 1H), 8.03(d, J=8.8 Hz, 2H), 7.94—7.87(m, 1H), 7.76(s, 1H), 7.71(d, J=8.8 Hz, 2H), 7.53(t, J=8.0 Hz, 1H), 7.29—7.20(m, 1H), 6.65(s, 1H), 3.63—3.53(m, 4H), 3.06(t, J=6.4 Hz, 2H), 2.93—2.79(m, 4H), 2.32(s, 3H), 2.08(s, 3H), 1.94—1.79(m, 1H), 0.89(d, J=6.7 Hz, 6H) | 163.9, 161.0, 160.0, 158.7, 158.2, 156.4, 141.9, 141.8, 134.7, 130.9, 129.4, 129.0, 122.5, 119.7, 119.4, 118.6, 116.5, 65.2, 48.7, 45.9, 27.8, 20.3, 16.4, 13.4 |
| 9b | 9.99(s, 1H), 8.72(s, 1H), 8.46(s, 1H), 8.40(s, 1H), 7.92(d, J=8.8 Hz, 2H), 7.87(d, J=0.7 Hz, 1H), 7.86—7.81(m, 1H), 7.78(d, J=8.8 Hz, 2H), 7.54(t, J=8.0 Hz, 1H), 7.28—7.21(m, 1H), 3.70—3.63(m, 4H), 3.58—3.52(m, 4H), 2.91—2.80(m, 4H), 2.43—2.37(m, 4H), 2.33(s, 3H), 2.22(s, 3H), 2.11(s, 3H) | 163.8, 160.3, 160.2, 158.6, 158.2, 156.3, 142.0, 141.5, 134.9, 131.3, 129.4, 129.2, 122.5, 119.9, 119.4, 118.6, 116.5, 104.2, 65.3, 54.5, 46.0, 45.8, 43.7, 16.4, 13.3 |
| 9c | 9.99(s, 1H), 8.74(s, 1H), 8.46(s, 1H), 8.42(s, 1H), 7.97—7.86(m, 3H), 7.85—7.73(m, 3H), 7.53(t, J=8.0 Hz, 1H), 7.27—7.21(m, 1H), 3.71—3.59(m, 8H), 3.58—3.50(m, 4H), 2.90—2.78(m, 4H), 2.33(s, 3H), 2.12(s, 3H) | 163.6, 160.3, 160.2, 158.6, 158.2, 156.1, 141.9, 141.4, 134.6, 131.3, 129.4, 129.3, 122.5, 119.8, 119.4, 118.6, 116.4, 104.6, 66.1, 65.2, 45.9, 44.4, 16.5, 13.4 |
| 9d | 9.99(s, 1H), 9.31(s, 1H), 8.64—8.53(m, 2H), 8.48(s, 1H), 8.01(s, 1H), 7.97—7.90(m, 4H), 7.77(d, J=8.8 Hz, 2H), 7.61—7.49(m, 2H), 7.27—7.17(m, 2H), 6.90—6.83(m, 1H), 3.61—3.50(m, 4H), 2.89—2.80(m, 4H), 2.34(s, 3H), 2.18(s, 3H) | 163.9, 160.1, 158.9, 158.2, 157.6, 155.7, 142.6, 141.9, 141.0, 134.7, 132.9, 132.0, 129.8, 129.4, 129.3, 122.5, 120.8, 120.0, 119.5, 118.7, 117.5, 116.8, 116.5, 107.0, 65.2, 45.9, 16.4, 13.6 |
| 9e | 9.97(s, 1H), 8.58(s, 1H), 8.45(s, 1H), 8.28(s, 1H), 7.98—7.83(m, 3H), 7.79(s, 1H), 7.64(d, J=8.6 Hz, 2H), 7.53(t, J=8.0 Hz, 1H), 7.34(dd, J=8.6, 5.7 Hz, 2H), 7.27—7.15(m, 2H), 7.10(t, J=8.9 Hz, 2H), 4.44(d, J=6.2 Hz, 2H), 3.61—3.50(m, 4H), 2.89—2.80(m, 4H), 2.31(s, 3H), 2.08(s, 3H) | 163.9, 160.9(d,J=241.5 Hz), 160.6, 160.0, 158.8, 158.1, 156.3, 141.9, 141.5, 137.1(d, J=2.9 Hz), 134.8, 131.0, 129.3, 128.9, 128.6(d, J=8.0 Hz), 122.4, 119.7, 119.4, 118.6, 116.5, 114.6(d, J=21.1 Hz), 103.9, 65.2, 45.9, 43.5, 16.3, 13.3 |
Table 2 1H NMR and 13C NMR data of compounds 3, 5, 6, 7a—7k, 8a—8c and 9a—9e
| Compd. | 1H NMR(300 MHz), δa | 13C NMR(75 MHz), δb |
|---|---|---|
| 3 | 8.60(d, J=0.7 Hz, 1H), 8.44—8.34(m, 2H), 7.89—7.79(m, 2H), 2.41(d, J=0.7 Hz, 3H) | 165.6, 162.1, 142.6, 130.2, 129.0, 127.1, 125.6, 123.9, 16.5 |
| 5 | 10.12(s,1H), 8.57(s,1H), 8.44(t, J=1.9 Hz,1H), 8.41—8.32(m, 2H), 8.02—7.91(m, 3H), 7.55(t, J=8.0 Hz, 1H), 7.29—7.23(m, 1H), 3.62—3.54(m, 4H), 2.87—2.79(m, 4H), 2.26(s, 3H) | 162.3, 160.5, 158.0, 147.6, 144.0, 141.4, 134.6, 130.0, 129.4, 123.2, 122.5, 119.6, 119.1, 116.6, 65.1, 45.8, 15.4 |
| 6 | 9.83(s, 1H), 8.61(s, 1H), 8.34(s, 1H), 7.94—7.80(m, 1H), 7.64—7.46(m, 3H), 7.32—7.13(m, 1H), 6.66(d, J=8.6 Hz, 2H), 5.58(s, 2H), 3.66—3.53(m, 4H), 2.90—2.82(m, 4H), 2.30(s, 3H) | 164.0, 159.5, 158.0, 150.1, 142.0, 134.6, 130.2, 129.2, 124.7, 122.2, 119.1, 117.8, 116.1, 112.8, 65.1, 45.8, 16.8 |
| 7a | 10.18(s, 1H), 10.00(s, 1H), 8.60(t, J=1.8 Hz, 1H), 8.48(s, 1H), 7.95—7.88(m, 1H), 7.75(s, 4H), 7.56(t, J=8.0 Hz, 1H), 7.30—7.24(m, 1H), 3.65—3.57(m, 4H), 2.91—2.84(m, 4H), 2.31(s, 3H), 2.12(s, 3H) | 168.7, 163.8, 160.1, 158.2, 141.9, 140.4, 134.7, 132.4, 129.6, 129.5, 122.6, 119.5, 118.7, 118.3, 116.5, 65.3, 46.0, 24.1, 16.3 |
| 7b | 10.08(s, 1H), 9.98(s, 1H), 8.57(s, 1H), 8.45(s, 1H), 7.91—7.85(m, 1H), 7.79—7.67(m, 4H), 7.54(t, J=8.0 Hz, 1H), 7.28—7.19(m, 1H), 3.62—3.52(m, 4H), 2.90—2.78(m, 4H), 2.28(s, 3H), 2.23(d, J=7.0 Hz, 2H), 2.18—2.02(m, 1H), 0.96(d, J=6.5 Hz, 6H) | 171.0, 163.8, 160.1, 158.2, 141.9, 140.4, 134.7, 132.4, 129.5, 129.4, 122.5, 119.5, 118.7, 118.4, 116.5, 65.3, 45.9, 45.7, 25.6, 22.3, 16.2 |
| 7c | 10.01(s, 1H), 9.97(s, 1H), 8.59—8.54(m, 1H), 8.45(s, 1H), 7.92—7.85(m, 1H), 7.79—7.66(m, 4H), 7.54(t, J=8.0 Hz, 1H), 7.27—7.21(m, 1H), 3.61—3.54(m, 4H), 2.88—2.79(m, 4H), 2.29(s, 3H), 2.24(s, 2H), 1.05(s, 9H) | 170.3, 163.7, 160.1, 158.1, 141.9, 140.2, 134.6, 132.4, 129.5, 129.4, 122.5, 119.5, 118.7, 118.5, 116.5, 65.2, 49.6, 45.9, 30.9, 29.6, 16.2 |
| 7d | 9.98(s, 1H), 9.90(s, 1H), 8.59—8.54(m, 1H), 8.46(s, 1H), 7.93—7.82(m, 3H), 7.72(d, J=8.7 Hz, 2H), 7.54(t, J=8.0 Hz, 1H), 7.27—7.21(m, 1H), 4.43(dd, J=8.1, 5.5 Hz, 1H), 4.08—3.95(m, 1H), 3.92—3.79(m, 1H), 3.65—3.50(m, 4H), 2.91—2.78(m, 4H), 2.28(s, 3H), 2.27—2.16(m, 1H), 2.09—1.95(m, 1H), 1.95—1.81(m, 2H) | 171.8, 163.8, 160.1, 158.1, 141.8, 139.4, 134.7, 133.0, 129.4, 129.3, 122.5, 119.5, 119.2, 118.7, 116.4, 77.9, 68.8, 65.2, 45.9, 29.9, 25.1, 16.2 |
| 7e | 10.39(s, 1H), 9.98(s, 1H), 8.57(s, 1H), 8.45(s, 1H), 7.92—7.85(m, 1H), 7.78—7.68(m, 4H), 7.53(t, J=8.0 Hz, 1H), 7.43—7.34(m, 2H), 7.27—7.10(m, 3H), 3.69(s, 2H), 3.61—3.52(m, 4H), 2.88—2.78(m, 4H), 2.28(s, 3H) | 169.3, 163.7, 161.1(d, J=242.1 Hz), 160.1, 158.1, 141.9, 140.2, 134.7, 132.7, 132.0(d, J=3.1 Hz), 131.0(d, J=8.0 Hz), 129.6, 129.4, 122.5, 119.5, 118.7, 118.5, 116.5, 115.0(d, J=21.2 Hz), 65.2, 45.9, 42.4, 16.2 |
| 7f | 10.88(s, 1H), 10.01(s, 1H), 8.82—8.76(m, 1H), 8.59—8.56(m, 1H), 8.49(s, 1H), 8.25—8.18(m, 1H), 8.15—8.06(m, 3H), 7.97—7.87(m, 1H), 7.79(d, J=8.7 Hz, 2H), 7.75—7.69(m, 1H), 7.55(t, J=8.0 Hz, 1H), 7.30—7.22(m, 1H), 3.66—3.54(m, 4H), 2.91—2.83(m, 4H), 2.32(s, 3H) | 169.1, 168.0, 165.4, 163.4, 155.0, 153.7, 147.1, 144.6, 143.4, 140.0, 138.6, 136.7, 134.7, 133.9, 132.3, 127.7, 124.9, 124.8, 124.1, 121.7, 70.5, 51.2, 21.4 |
| 7g | 10.68(s, 1H), 10.03(s, 1H), 9.27—9.08(m, 1H), 8.88—8.76(m, 1H), 8.62(s, 1H), 8.51(s, 1H), 8.40—8.30(m, 1H), 8.00—7.90(m, 3H), 7.82(d, J=8.7 Hz, 2H), 7.66—7.51(m,2H), 7.34—7.22(m,1H), 3.73—3.52(m, 4H), 2.99—2.79(m, 4H), 2.34(s, 3H) | 164.3, 163.7, 160.2, 158.2, 152.2, 148.8, 141.9, 139.9, 135.6, 134.6, 133.4, 130.5, 129.5, 129.4, 123.5, 122.6, 119.7, 119.5, 118.8, 116.5, 65.3, 46.0, 16.2 |
| 7h | 11.79(s, 1H), 9.98(s, 1H), 9.94(s, 1H), 8.67—8.59(m, 1H), 8.47(s, 1H), 8.36(d, J=3.0 Hz, 1H), 8.27—8.19(m, 1H), 8.00—7.87(m, 3H), 7.78(d, J=8.7 Hz, 2H), 7.61—7.46(m, 2H), 7.29—7.13(m, 3H), 3.64—3.52(m, 4H), 2.93—2.79(m, 4H), 2.33(s, 3H) | 163.9, 163.4, 160.1, 158.2, 141.9, 141.0, 136.2, 134.7, 131.9, 129.5, 129.0, 128.7, 126.4, 122.5, 122.2, 121.1, 120.8, 119.5, 118.9, 118.7, 116.5, 112.0, 110.4, 65.3, 45.9, 16.3 |
| 7i | 9.96(s, 1H), 8.91(s, 1H), 8.64(s, 1H), 8.60—8.53(m,1H), 8.45(s, 1H), 7.94—7.87(m, 1H), 7.72(d, J=8.7 Hz, 2H), 7.64—7.49(m, 3H), 7.37(d, J=8.4 Hz, 2H), 7.26—7.21(m, 1H), 7.10(d, J=8.4 Hz, 2H), 3.62—3.56(m, 4H), 2.89—2.82(m, 4H), 2.30(s, 3H), 2.25(s, 3H) | 163.9, 160.1, 158.2, 152.5, 141.9, 141.1, 137.0, 134.7, 131.1, 130.9, 129.7, 129.4, 129.2, 122.5, 119.5, 118.7, 118.4, 117.4, 116.5, 65.3, 46.0, 20.4, 16.4 |
| 7j | 8.54—8.48(m, 1H), 8.36(s, 1H), 8.18(d, J=5.8 Hz, 1H), 7.83(s, 1H), 7.73(d, J=8.5 Hz, 2H), 7.68—7.60(m, 1H), 7.57—7.43(m,4H), 7.42—7.33(m, 1H), 6.71(d, J=5.8 Hz, 1H), 3.73—3.65(m, 4H), 3.06—2.95(m, 4H), 2.34(s, 3H) | 163.6, 161.2, 160.1, 159.3, 158.1, 157.3, 141.8, 139.9, 134.6, 132.6, 129.7, 129.4, 122.5, 119.5, 119.2, 118.7, 116.4, 106.4, 65.2, 45.9, 16.2 |
| 7k | 10.00(s, 1H), 9.03(s, 1H), 8.60(s, 1H), 8.48(s, 1H), 8.12(s, 1H), 7.96—7.73(m, 5H), 7.54(t, J=8.0 Hz, 1H), 7.25(d, 1H), 3.67—3.53(m, 4H), 2.94—2.76(m, 4H), 2.32(s, 3H), 2.23(s, 3H) | 163.7, 160.1, 160.0, 158.2, 156.7, 156.6, 141.9, 139.8, 134.7, 133.0, 129.4, 129.2, 122.5, 121.1, 119.5, 118.7, 116.4, 114.9, 65.3, 45.9, 16.3, 13.5 |
| 8a | 9.94(s, 1H), 9.42(s, 1H), 8.54(s, 1H), 8.42(s, 1H), 7.95—7.83(m, 4H), 7.69(d, J=8.7 Hz, 2H), 7.51(t, J=8.0 Hz, 1H), 7.27—7.20(m, 1H), 6.95(s, 1H), 6.02(d, J=5.7 Hz, 1H), 3.60—3.50(m, 4H), 3.08(t, J=6.4 Hz, 2H), 2.89—2.75(m, 4H), 2.29(s, 3H), 1.95—1.80(m, 1H), 0.89(d, J=6.7 Hz, 6H) | 163.9, 162.0, 160.4, 160.0, 158.2, 156.5, 156.3, 141.9, 134.7, 130.6, 129.4, 126.4, 122.5, 119.4, 118.6, 118.3, 117.3, 116.5, 65.3, 48.6, 45.9, 27.8, 20.3, 16.4 |
| 8b | 9.99(s, 1H), 9.55(s, 1H), 8.75(s, 1H), 8.45(s, 1H), 7.99(d, J=5.6 Hz, 1H), 7.88—7.74(m, 5H), 7.54(t, J=8.0 Hz, 1H), 7.30—7.21(m, 1H), 6.12(d, J=5.6 Hz, 1H), 3.79—3.68(m, 4H), 3.62—3.51(m, 4H), 2.91—2.80(m, 4H), 2.46—2.38(m, 4H), 2.34(s, 3H), 2.23(s, 3H) | 163.5, 161.1, 160.1, 160.0, 158.1, 156.2, 141.9, 141.5, 134.8, 130.7, 129.6, 129.2, 122.4, 119.3, 118.4, 118.3, 116.3, 97.0, 65.2, 54.4, 45.8, 45.7, 43.4, 16.4 |
| 8c | 9.99(s, 1H), 9.58(s, 1H), 8.77(s, 1H), 8.45(s, 1H), 8.01(d, J=5.6 Hz, 1H), 7.90—7.75(m, 5H), 7.53(t, J=8.0 Hz, 1H), 7.30—7.21(m, 1H), 6.15(d, J=5.6 Hz, 1H), 3.70(s, 8H), 3.59—3.50(m, 4H), 2.91—2.81(m, 4H), 2.34(s, 3H) | 163.4, 161.3, 160.3, 160.2, 158.1, 156.3, 141.9, 141.5, 134.6, 130.8, 129.7, 129.4, 122.5, 119.4, 118.5, 118.3, 116.3, 97.6, 66.1, 65.2, 45.9, 44.2, 16.5 |
| 9a | 9.97(s, 1H), 8.59(t, J=1.7 Hz, 1H), 8.45(s, 1H), 8.26(s, 1H), 8.03(d, J=8.8 Hz, 2H), 7.94—7.87(m, 1H), 7.76(s, 1H), 7.71(d, J=8.8 Hz, 2H), 7.53(t, J=8.0 Hz, 1H), 7.29—7.20(m, 1H), 6.65(s, 1H), 3.63—3.53(m, 4H), 3.06(t, J=6.4 Hz, 2H), 2.93—2.79(m, 4H), 2.32(s, 3H), 2.08(s, 3H), 1.94—1.79(m, 1H), 0.89(d, J=6.7 Hz, 6H) | 163.9, 161.0, 160.0, 158.7, 158.2, 156.4, 141.9, 141.8, 134.7, 130.9, 129.4, 129.0, 122.5, 119.7, 119.4, 118.6, 116.5, 65.2, 48.7, 45.9, 27.8, 20.3, 16.4, 13.4 |
| 9b | 9.99(s, 1H), 8.72(s, 1H), 8.46(s, 1H), 8.40(s, 1H), 7.92(d, J=8.8 Hz, 2H), 7.87(d, J=0.7 Hz, 1H), 7.86—7.81(m, 1H), 7.78(d, J=8.8 Hz, 2H), 7.54(t, J=8.0 Hz, 1H), 7.28—7.21(m, 1H), 3.70—3.63(m, 4H), 3.58—3.52(m, 4H), 2.91—2.80(m, 4H), 2.43—2.37(m, 4H), 2.33(s, 3H), 2.22(s, 3H), 2.11(s, 3H) | 163.8, 160.3, 160.2, 158.6, 158.2, 156.3, 142.0, 141.5, 134.9, 131.3, 129.4, 129.2, 122.5, 119.9, 119.4, 118.6, 116.5, 104.2, 65.3, 54.5, 46.0, 45.8, 43.7, 16.4, 13.3 |
| 9c | 9.99(s, 1H), 8.74(s, 1H), 8.46(s, 1H), 8.42(s, 1H), 7.97—7.86(m, 3H), 7.85—7.73(m, 3H), 7.53(t, J=8.0 Hz, 1H), 7.27—7.21(m, 1H), 3.71—3.59(m, 8H), 3.58—3.50(m, 4H), 2.90—2.78(m, 4H), 2.33(s, 3H), 2.12(s, 3H) | 163.6, 160.3, 160.2, 158.6, 158.2, 156.1, 141.9, 141.4, 134.6, 131.3, 129.4, 129.3, 122.5, 119.8, 119.4, 118.6, 116.4, 104.6, 66.1, 65.2, 45.9, 44.4, 16.5, 13.4 |
| 9d | 9.99(s, 1H), 9.31(s, 1H), 8.64—8.53(m, 2H), 8.48(s, 1H), 8.01(s, 1H), 7.97—7.90(m, 4H), 7.77(d, J=8.8 Hz, 2H), 7.61—7.49(m, 2H), 7.27—7.17(m, 2H), 6.90—6.83(m, 1H), 3.61—3.50(m, 4H), 2.89—2.80(m, 4H), 2.34(s, 3H), 2.18(s, 3H) | 163.9, 160.1, 158.9, 158.2, 157.6, 155.7, 142.6, 141.9, 141.0, 134.7, 132.9, 132.0, 129.8, 129.4, 129.3, 122.5, 120.8, 120.0, 119.5, 118.7, 117.5, 116.8, 116.5, 107.0, 65.2, 45.9, 16.4, 13.6 |
| 9e | 9.97(s, 1H), 8.58(s, 1H), 8.45(s, 1H), 8.28(s, 1H), 7.98—7.83(m, 3H), 7.79(s, 1H), 7.64(d, J=8.6 Hz, 2H), 7.53(t, J=8.0 Hz, 1H), 7.34(dd, J=8.6, 5.7 Hz, 2H), 7.27—7.15(m, 2H), 7.10(t, J=8.9 Hz, 2H), 4.44(d, J=6.2 Hz, 2H), 3.61—3.50(m, 4H), 2.89—2.80(m, 4H), 2.31(s, 3H), 2.08(s, 3H) | 163.9, 160.9(d,J=241.5 Hz), 160.6, 160.0, 158.8, 158.1, 156.3, 141.9, 141.5, 137.1(d, J=2.9 Hz), 134.8, 131.0, 129.3, 128.9, 128.6(d, J=8.0 Hz), 122.4, 119.7, 119.4, 118.6, 116.5, 114.6(d, J=21.1 Hz), 103.9, 65.2, 45.9, 43.5, 16.3, 13.3 |
| Compd. | Inhibitiona(%)(10 μmol/L) | IC50/(μmol·L-1) | Compd. | Inhibitiona(%)(10 μmol/L) | IC50/(μmol·L-1) |
|---|---|---|---|---|---|
| 7a | 75 | 6.4 | 7k | 52 | 3.5 |
| 7b | 51 | 9.1 | 8a | 17 | NTb |
| 7c | 55 | 4.3 | 8b | 0 | NTb |
| 7d | 31 | NTb | 8c | 95 | 0.224 |
| 7e | 35 | NTb | 9a | 17 | NTb |
| 7f | 17 | NTb | 9b | 0 | NTb |
| 7g | 78 | 1.0 | 9c | 97 | 0.113 |
| 7h | 76 | 2.1 | 9d | 3 | NTb |
| 7i | 44 | NTb | 9e | 0 | NTb |
| 7j | 72 | 8.4 |
Table 3 Antitumor activities against human breast cancer cell line(MCF-7)
| Compd. | Inhibitiona(%)(10 μmol/L) | IC50/(μmol·L-1) | Compd. | Inhibitiona(%)(10 μmol/L) | IC50/(μmol·L-1) |
|---|---|---|---|---|---|
| 7a | 75 | 6.4 | 7k | 52 | 3.5 |
| 7b | 51 | 9.1 | 8a | 17 | NTb |
| 7c | 55 | 4.3 | 8b | 0 | NTb |
| 7d | 31 | NTb | 8c | 95 | 0.224 |
| 7e | 35 | NTb | 9a | 17 | NTb |
| 7f | 17 | NTb | 9b | 0 | NTb |
| 7g | 78 | 1.0 | 9c | 97 | 0.113 |
| 7h | 76 | 2.1 | 9d | 3 | NTb |
| 7i | 44 | NTb | 9e | 0 | NTb |
| 7j | 72 | 8.4 |
| [1] | Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S., Science, 2002, 298(5600), 1912—1934 |
| [2] | Zhang J. M., Yang P. L., Gray N. S., Nat. Rev. Cancer, 2009, 9, 28—39 |
| [3] | Lamontanaral A. J., Gencer E. B., Kuzyk O., Hantsche O., Biochim. Biophys. Acta, 2013, 1834(7), 1449—1459 |
| [4] | Sun X. X., Sun T., Wang T. Y., Zhang Y., Liu H. J., Wang Q., Niu G. J., Liu W., Zhou H. G., Yang C., Chem. Res. Chinese Universities, 2013, 29(6), 1098—1103 |
| [5] | Zhang C., Bollag G., Curr. Opin. Genet. Dev., 2010, 20, 79—86 |
| [6] | Liu Y., Gray N. S., Nat. Chem. Biol., 2006, 2, 358—364 |
| [7] | Wu B., Wang H. L., Pettus L. P., Wurz R. P., Doherty E. M., Henkl B., McBride H. J., Saris C. J. M., Wong L. M., Plant M. H., Sherman L., Lee M. R., Hsieh Faye., Tasker A. S., J. Med. Chem., 2010, 53, 6398—6411 |
| [8] | Liu C. J., Lin J., Wrobleski S. T., Lin S. Q., Hynes J. Jr., Wu H., Dyckman A. J., Li T. L., Wityak J., Gillooly K. M., Pitt S., Shen D. R., Zhang R. F., McIntyre K. W., Salter-Cid L., Shuster D. J., Zhang H. J., Marathe P. H., Doweyko A. M., Sack J. S., Kiefer S. E., Kish F. K., Newitt J. A., McKinnon M., Dodd J. H., Barrish J. C., Schieve G. L., Lefthetris K., J. Med. Chem., 2010, 53, 6629—6639 |
| [9] | Nagashima K., Shumway S. D., Sathyanarayanan S., Chen A. H., Dolinski B., Xu Y. Y., Keilhack H., Nguyen T., Wiznerowicz M., Li L. X., Lutterbach B. A., Chi A., Paweletz C., Allison T., Yan Y. W., Munshi S. K., Klippel A., Kraus M., Bobkova E. V., Deshmukh S., Xu Z. W., Mueller U., Szewczak A. A., Pan B. S., Richon V., Pollock R., Jensen P. B., Northrup A., Andersen J. N., J. Biol. Chem., 2011, 286, 6433—6448 |
| [10] | Dietrich J., Hulme C., Hurley L. H., Bioorg. Med. Chem., 2010, 18, 5738—5748 |
| [11] | Nagar B., Bornmann W. G., Pellicena P., Schindler T., Veach D. R., Miller W. T., Clarkson B., Kuriyan J., Cancer Res., 2002, 62, 4236—4243 |
| [12] | Xing L., Shieh H. S., Selness S. R., Devraj R. V., Walker J. K., Devadas B., Hope H. R., Compton R. P., Schindler J. F., Hirsch J. L., Benson A. G., Kurumbail R. G., Stegeman R. A., Williams J. M., Broadus R. M., Walden Z., Monahan J. B., Biochem., 2009, 48, 6402—6411 |
| [13] | Yang H. L., Xu G. X., Bao M. Y., Zhang D. P., Li Z. W., Pei Y. Z., Chem. J. Chinese Universities, 2014, 35(12), 2584—2592 |
| (杨洪亮, 徐国兴, 宝梅英, 张大鹏, 李志伟, 裴亚中. 高等学校化学学报, 2014, 35(12), 2584—2592) | |
| [14] | Miyaura N., Suzuki A., Chem. Rev., 1995, 95(7), 2457—2483 |
| [15] | Baudoin O., Guenard D., Gueritte F., J. Org. Chem., 2000, 65(26), 9268—9271 |
| [16] | Zhu L., Duquette J., Zhang M., J. Org. Chem., 2003, 68(9), 3729—3732 |
| [17] | Qandil A. M., Hassan M. A., Al-Shar'iNzar A., Arch. Pharm., 2008, 341, 99—112 |
| [18] | Yapi A. D., Desbois N., Chezal J. M., Chavignon O., Teulade J. C., Valentin A., Blache Y., Eur. J. Med. Chem., 2010, 45, 2854—2859 |
| [19] | Tim M., J. Immunol. Methods, 1983, 68(1), 55—63 |
| [20] | Wang S. D., Griffiths G., Midgley C. A., Barnett A. L., Cooper M., Grabarek J., Ingram L., Jackson W., Kontopidis G., McClue S. J., McInnes C., McLachlan J., Meades C., Mezna M., Stuart I., Thomas M. P., Zheleva D. I., Lane D. P., Jackson R. C., Glover D. M., Blake D. G., Fischer P. M., Chem. Bio., 2010, 17, 1111—1121 |
| [21] | Reddy S. V., Rao G. M., Kumar B. V., Reddy K. N., Sravya K., Goverdhan P., Rathore V., Deora G. S., Pal M., Med. Chem. Commun., 2014, 5, 587—592 |
| [22] | Badrinarayan P., Sastry G. N., J. Mol. Grap. Model, 2012, 34, 89—100 |
| [23] | Bogoyevitch M. A., Fairlie D. P., Drug Discov. Today, 2007, 12, 622—633 |
| [1] | 肖艳华, 张广杰, 宗良, 刘国宏, 任丽君, 董俊兴. 开口箭化学成分及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(9): 1897. |
| [2] | 吕明君, 李雯, 杨新颖, 方浩. N9位芳基取代嘌呤-8-酮类衍生物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(2): 254. |
| [3] | 方芳,薛良敏,丛婧,田超,王孝伟,刘俊义,张志丽. 2-位或4-位取代吡啶并嘧啶类非经典叶酸拮抗剂的合成及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(10): 2111. |
| [4] | 张培全,杨倩倩,龙惠丹,陈鑫. 金诺芬衍生物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(10): 2097. |
| [5] | 刘莉, 马洋洋, 王宽, 贾云静, 李婉, 朱华结. β-咔啉衍生物的抗肿瘤及抗菌活性[J]. 高等学校化学学报, 2018, 39(4): 674. |
| [6] | 王磊, 郑国钧, 季奇, 陈博, 巩龙龙, 高聪敏, 杜镇建, 张兴民. PI3K/mTOR抑制剂的合成及生物活性[J]. 高等学校化学学报, 2017, 38(9): 1590. |
| [7] | 唐光辉, 张娅, 张玉萍, 周朋朋, 林治华, 王远强. 含磷嘧啶类CDK9抑制剂的分子对接、3D-QSAR和分子动力学模拟[J]. 高等学校化学学报, 2017, 38(11): 2061. |
| [8] | 白信法, 马宣, 解晓霞, 邵明莎, 郭宁宁, 严宁, 姚雷. 微管菌素类似物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2017, 38(1): 47. |
| [9] | 郭亮, 曹日晖, 范文玺, 甘紫云, 马芹. 双-β-咔啉衍生物的设计、合成及抗肿瘤活性[J]. 高等学校化学学报, 2016, 37(6): 1093. |
| [10] | 史蕾, 杨文聪, 曾淑莹, 莫婷婷, 张召, 曹曼丽, 刘海洋. 咔咯钴(Ⅲ)配合物与DNA的相互作用及抗肿瘤活性[J]. 高等学校化学学报, 2016, 37(6): 1059. |
| [11] | 张洁, 周昌健, 谢建伟, 代斌. 大黄酸-缬氨酸加合物的合成及初步抗肿瘤活性[J]. 高等学校化学学报, 2016, 37(12): 2159. |
| [12] | 王刚, 韩雷强, 方浩. 苯基哌嗪衍生物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2015, 36(12): 2435. |
| [13] | 郭华, 杨承玲, 王蔚, 赖全勇, 袁直. 基于海藻酸钠衍生物的肝靶向纳米前药的构建及抗肿瘤活性研究[J]. 高等学校化学学报, 2014, 35(8): 1835. |
| [14] | 童春义, 唐凤霞, 刘斌, 廖红东, 刘选明. 氟尿嘧啶-壳寡糖/硒纳米微球的制备及抑制肿瘤细胞生长活性[J]. 高等学校化学学报, 2014, 35(7): 1603. |
| [15] | 李小六, 马东来, 杨海龙, 谭官海, 杜会茹, 王克让, 张平竹, 陈华. 吖啶-多胺类缀合物的合成、 抗肿瘤活性及与DNA键合作用[J]. 高等学校化学学报, 2014, 35(6): 1181. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||