

高等学校化学学报 ›› 2016, Vol. 37 ›› Issue (2): 254.doi: 10.7503/cjcu20150716
王亮1,2( ), 瞿星1, 李站1, 顾昌翰1, 胡思前1,2(
), 瞿星1, 李站1, 顾昌翰1, 胡思前1,2( )
)
收稿日期:2015-09-14
出版日期:2016-02-10
发布日期:2016-01-04
作者简介:联系人简介: 王 亮, 男, 博士, 讲师, 主要从事杂环化合物的C—H官能化研究. E-mail:基金资助:
WANG Liang1,2,*( ), QU Xing1, LI Zhan1, GU Changhan1, HU Siqian1,2,*(
), QU Xing1, LI Zhan1, GU Changhan1, HU Siqian1,2,*( )
)
Received:2015-09-14
Online:2016-02-10
Published:2016-01-04
Contact:
WANG Liang,HU Siqian
E-mail:wangliang@jhun.edu.cn;husiqian@126.com
Supported by:摘要:
研究了无金属参与的二乙酸碘苯和吲哚的C3—H乙酸化反应, 通过对取代基效应、 温度以及二乙酸碘苯用量等因素的考察, 建立了反应的最佳条件: 反应温度60 ℃, 乙酸(HOAc)为溶剂. 在无需任何添加剂条件下, 以中等到良好的收率获得一系列C3位乙酸化的吲哚衍生物. 采用红外光谱、 核磁共振波谱、 高分辨质谱及X射线单晶衍射分析对目标化合物进行了结构表征, 并推测了可能的反应机理. 该催化体系对于克量级规模反应均能获得很好的结果.
中图分类号:
TrendMD:
王亮, 瞿星, 李站, 顾昌翰, 胡思前. 无金属参与的二乙酸碘苯促进吲哚C3—H乙酸化反应. 高等学校化学学报, 2016, 37(2): 254.
WANG Liang, QU Xing, LI Zhan, GU Changhan, HU Siqian. Metal-free C3—H Acetoxylation of Indoles Promoted by PhI(OAc)2†. Chem. J. Chinese Universities, 2016, 37(2): 254.
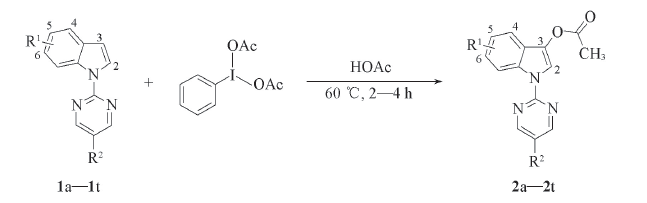
Scheme 1 Synthetic route for C3—H acetoxylation of indoles 2a: R1=H, R2=H; 2b: R1=4-Me, R2=H; 2c: R1=4-OBn, R2=H; 2d: R1=4-Cl, R2=H; 2e: R1=4-CO2Me, R2=H; 2f: R1=5-Me, R2=H; 2g: R1=5-OMe, R2=H; 2h: R1=5-OBn, R2=H; 2i: R1=5-OPh, R2=H; 2j: R1=5-Cl, R2=H; 2k: R1=5-Br, R2=H; 2l: R1=6-Me, R2=H; 2m: R1=6-CO2Me, R2=H; 2n: R1=6-F, R2=H; 2o: R1=6-Cl, R2=H; 2p: R1=6-Br, R2=H; 2q: R1=5,6-Ph, R2=H; 2r: R1=H, R2=Me; 2s: R1=H, R2=Ph; 2t: R1=H, R2=Br
| Compd. | Appearance | Yield*(%) | m. p./℃ | HRMS(calcd.), m/z [M+Na+] | IR(KBr), |
|---|---|---|---|---|---|
| 2a | White solid | 85 | 123—124 | 276.0747(276.0743) | 3195, 1743, 1573, 1458, 1428, 1369, 1316, 1208, 1158, 1124, 1073,1015 |
| 2b | Yellow solid | 76 | 143—144 | 290.0925(290.0900) | 3203, 2923, 2854, 1753, 1568, 1433, 1354, 1300, 1198, 1147 |
| 2c | White solid | 63 | 127—128 | 382.1155(382.1162) | 3210, 1738, 1573, 1499, 1448, 1362, 1271, 1232, 1160, 1096, 1064, 913 |
| 2d | White solid | 73 | 155—156 | 310.0352(310.0354) | 3192, 1756, 1563, 1452, 1360, 1309, 1212, 1001, 891, 770 |
| 2e | White solid | 75 | 134—135 | 334.0789(334.0798) | 2955, 1749, 1713, 1570, 1357, 1283, 1190, 1091, 1020, 954, 902, 740 |
| 2f | White solid | 78 | 165—166 | 290.0900(290.0900) | 3201, 1746, 1581, 1456, 1426, 1353, 1202, 1147, 1122, 864 |
| 2g | Yellow solid | 80 | 166—167 | 306.0849(306.0849) | 3209, 1744, 1536, 1436, 1366, 1210, 1075, 1020, 905, 801, 766, 747 |
| 2h | White solid | 65 | 166—167 | 382.1128(382.1162) | 3194, 1749, 1576, 1479, 1453, 1296, 1203, 1078, 1013, 919 |
| 2i | White solid | 86 | 139—140 | 368.1009(368.1006) | 3219, 1747, 1565, 1471, 1421, 1361, 1289, 1198, 1072, 965, 922 |
| 2j | Yellowish solid | 83 | 181—182 | 310.0335(310.0354) | 3206, 1756, 1573, 1459, 1373, 1346, 1292, 1203, 1065 |
| 2k | Yellowish solid | 85 | 182—183 | 353.9846(353.9846) | 3023, 1787, 1735, 1456, 1353, 1147, 1014, 919, 855, 784 |
| 2l | White solid | 83 | 153—154 | 290.0875(290.0900) | 3195, 2971, 2856, 1742, 1573, 1436, 1368, 1321, 1212, 1131, 789 |
| 2m | Yellow solid | 63 | 199—200 | 334.0896(334.0798) | 3197, 1752, 1717, 1571, 1441, 1373, 1280, 1210, 1140, 1064, 990 |
| 2n | Reddish solid | 77 | 167—168 | 294.0660(294.0649) | 3197, 1745, 1575, 1441, 1370, 1208, 1104, 976, 905, 855, 792 |
| 2o | White solid | 70 | 179—180 | 310.0339(310.0354) | 3199, 1793, 1744, 1440, 1367, 1209, 1129, 1066, 957, 792, 506 |
| 2p | White solid | 80 | 178—179 | 353.9838(353.9849) | 3202, 1746, 1577, 1441, 1367, 1209, 1128, 1080, 1001, 954, 786 |
| 2q | White solid | 45 | 155—157 | 326.0901(326.0900) | 3211, 1746, 1571, 1459, 1427, 1363, 1297, 1220, 1130, 1023, 883, 775 |
| 2r | Yellowish solid | 84 | 158—159 | 290.0900(290.0900) | 3207, 1745, 1598, 1556, 1444, 1366, 1317, 1207, 1008, 742 |
| 2s | White solid | 81 | 133—134 | 352.1062(352.1056) | 3195, 1752, 1589, 1548, 1444, 1366, 1197, 1123, 1009, 887, 746 |
| 2t | White solid | 72 | 182—183 | 353.9681(353.9849) | 3199, 1746, 1598, 1557, 1440, 1317, 1207, 1116, 1010, 743 |
Table 1 Appearance, yields, melting points, HRMS and IR data of compounds 2a—2t
| Compd. | Appearance | Yield*(%) | m. p./℃ | HRMS(calcd.), m/z [M+Na+] | IR(KBr), |
|---|---|---|---|---|---|
| 2a | White solid | 85 | 123—124 | 276.0747(276.0743) | 3195, 1743, 1573, 1458, 1428, 1369, 1316, 1208, 1158, 1124, 1073,1015 |
| 2b | Yellow solid | 76 | 143—144 | 290.0925(290.0900) | 3203, 2923, 2854, 1753, 1568, 1433, 1354, 1300, 1198, 1147 |
| 2c | White solid | 63 | 127—128 | 382.1155(382.1162) | 3210, 1738, 1573, 1499, 1448, 1362, 1271, 1232, 1160, 1096, 1064, 913 |
| 2d | White solid | 73 | 155—156 | 310.0352(310.0354) | 3192, 1756, 1563, 1452, 1360, 1309, 1212, 1001, 891, 770 |
| 2e | White solid | 75 | 134—135 | 334.0789(334.0798) | 2955, 1749, 1713, 1570, 1357, 1283, 1190, 1091, 1020, 954, 902, 740 |
| 2f | White solid | 78 | 165—166 | 290.0900(290.0900) | 3201, 1746, 1581, 1456, 1426, 1353, 1202, 1147, 1122, 864 |
| 2g | Yellow solid | 80 | 166—167 | 306.0849(306.0849) | 3209, 1744, 1536, 1436, 1366, 1210, 1075, 1020, 905, 801, 766, 747 |
| 2h | White solid | 65 | 166—167 | 382.1128(382.1162) | 3194, 1749, 1576, 1479, 1453, 1296, 1203, 1078, 1013, 919 |
| 2i | White solid | 86 | 139—140 | 368.1009(368.1006) | 3219, 1747, 1565, 1471, 1421, 1361, 1289, 1198, 1072, 965, 922 |
| 2j | Yellowish solid | 83 | 181—182 | 310.0335(310.0354) | 3206, 1756, 1573, 1459, 1373, 1346, 1292, 1203, 1065 |
| 2k | Yellowish solid | 85 | 182—183 | 353.9846(353.9846) | 3023, 1787, 1735, 1456, 1353, 1147, 1014, 919, 855, 784 |
| 2l | White solid | 83 | 153—154 | 290.0875(290.0900) | 3195, 2971, 2856, 1742, 1573, 1436, 1368, 1321, 1212, 1131, 789 |
| 2m | Yellow solid | 63 | 199—200 | 334.0896(334.0798) | 3197, 1752, 1717, 1571, 1441, 1373, 1280, 1210, 1140, 1064, 990 |
| 2n | Reddish solid | 77 | 167—168 | 294.0660(294.0649) | 3197, 1745, 1575, 1441, 1370, 1208, 1104, 976, 905, 855, 792 |
| 2o | White solid | 70 | 179—180 | 310.0339(310.0354) | 3199, 1793, 1744, 1440, 1367, 1209, 1129, 1066, 957, 792, 506 |
| 2p | White solid | 80 | 178—179 | 353.9838(353.9849) | 3202, 1746, 1577, 1441, 1367, 1209, 1128, 1080, 1001, 954, 786 |
| 2q | White solid | 45 | 155—157 | 326.0901(326.0900) | 3211, 1746, 1571, 1459, 1427, 1363, 1297, 1220, 1130, 1023, 883, 775 |
| 2r | Yellowish solid | 84 | 158—159 | 290.0900(290.0900) | 3207, 1745, 1598, 1556, 1444, 1366, 1317, 1207, 1008, 742 |
| 2s | White solid | 81 | 133—134 | 352.1062(352.1056) | 3195, 1752, 1589, 1548, 1444, 1366, 1197, 1123, 1009, 887, 746 |
| 2t | White solid | 72 | 182—183 | 353.9681(353.9849) | 3199, 1746, 1598, 1557, 1440, 1317, 1207, 1116, 1010, 743 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
|---|---|---|
| 2a | 8.81(d,J=8.4 Hz, 1H), 8.67(d, J=4.8 Hz, 2H), 8.43(s, 1H), 7.56(d, J=7.8 Hz, 1H), 7.37(t, J=7.5 Hz, 1H), 7.29—7.25(m, 1H), 7.02(t, J=4.8 Hz, 1H), 2.40(s, 3H) | 168.2, 158.1, 157.8, 133.5, 132.8, 124.6, 124.0, 122.2, 117.5, 116.4, 116.1, 114.6, 21.1 |
| 2b | 8.73—8.59(m, 3H), 8.36(s, 1H), 7.24(dd, J=10.3, 5.1 Hz, 1H), 7.02—6.92(m, 2H), 2.63(s, 3H), 2.37(s, 3H) | 168.7, 158.1, 157.7, 134.3, 133.2, 129.4, 124.5, 123.9, 122.7, 116.0, 114.8, 114.1, 29.7, 21.3, 18.8 |
| 2c | 8.66(d,J=4.8 Hz, 2H), 8.44(d, J=8.4 Hz, 1H), 8.06(s, 1H), 7.54—7.48(m, 2H), 7.40(dd, J=9.7, 5.6, 1.4 Hz, 3H), 7.25(d, J=4.6 Hz, 1H), 7.02(t, J=4.8 Hz, 1H), 6.74(d, J=7.9 Hz, 1H), 5.11(s, 2H), 1.91(s, 3H) | 169.8, 158.1, 157.7, 152.0, 136.9, 135.1, 133.2, 128.5, 128.4, 128.2, 125.4, 116.2, 114.5, 114.2, 110.0, 104.1, 70.4, 20.4 |
| 2d | 8.79—8.72(m, 1H), 8.66—8.61(m, 2H), 8.27(d, J=2.0 Hz, 1H), 7.20(t, J=7.7 Hz, 2H), 7.02(t, J=4.8, 3.4 Hz, 1H), 2.39(s, 3H) | 169.5, 158.1, 157.2, 134.6, 132.2, 125.0, 124.2, 123.3, 121.4, 116.9, 116.6, 115.3, 21.1 |
| 2e | 9.12(d,J=8.5 Hz, 1H), 8.66(d, J=4.7 Hz, 2H), 8.31(s, 1H), 7.82(d, J=7.5 Hz, 1H), 7.37(t, J=8.0 Hz, 1H), 7.05(t, J=4.6 Hz, 1H), 3.91(d, J=13.3 Hz, 3H), 2.41(s, 3H) | 170.1, 167.2, 158.2, 157.3, 134.5, 132.8, 125.4, 123.5, 123.0, 122.5, 120.7, 118.7, 116.7, 52.2, 21.0 |
| 2f | 8.69—8.59(m, 3H), 8.36(s, 1H), 7.33(s, 1H), 7.18(d, J=8.5 Hz, 1H), 6.97(t, J=4.2 Hz, 1H), 2.47(s, 3H), 2.39(s, 3H) | 168.2, 158.1, 157.7, 133.3, 131.7, 131.2, 126.1, 124.2, 117.2, 116.2, 115.9, 114.6, 21.4, 21.1 |
| 2g | 8.66(dd,J=22.8, 6.8 Hz, 3H), 8.39(s, 1H), 6.98(d, J=8.2 Hz, 3H), 3.89(s, 3H), 2.40(s, 3H) | 168.1, 158.1, 157.6, 155.6, 133.4, 127.8, 124.7, 117.5, 115.8, 115.1, 114.0, 99.4, 55.7, 21.1 |
| 2h | 8.70(d,J=8.7 Hz, 1H), 8.63(d, J=4.7 Hz, 2H), 8.40(s, 1H), 7.49(d, J=7.5 Hz, 2H), 7.40(t, J=7.3 Hz, 2H), 7.36—7.30(m, 1H), 7.06(d, J=9.8 Hz, 2H), 6.98(t, J=4.6 Hz, 1H), 5.13(s, 2H), 2.39(s, 3H), 5.13(s, 2H), 2.39(s, 3H) | 168.1, 158.1, 157.6, 154.8, 137.3, 133.4, 128.6, 127.9, 127.7, 124.7, 117.6, 115.9, 115.2, 114.5, 101.0, 70.6, 21.1 |
| 2i | 8.83—8.76(m, 1H), 8.67(d, J=4.8 Hz, 2H), 8.46(s, 1H), 7.38—7.29(m, 2H), 7.20(d, J=2.4 Hz, 1H), 7.14—6.99(m, 5H), 2.35(s, 3H) | 168.1, 158.6, 158.1, 157.6, 152.0, 133.3, 129.7, 129.5, 125.0, 122.5, 117.8, 117.6, 116.1, 115.7, 107.9, 21.0 |
| 2j | 8.84—8.61(m, 3H), 8.44(s, 1H), 7.52(s, 1H), 7.36—7.24(m, 1H), 7.05(s, 1H), 2.39(s, 3H) | 168.0, 158.2, 157.5, 132.6, 131.1, 127.9, 125.1, 124.8, 117.7, 117.1, 116.4, 115.9, 21.0 |
| 2k | 8.67(t,J=7.2 Hz, 3H), 8.42(s, 1H), 7.68(s, 1H), 7.43(d, J=8.9 Hz, 1H), 7.04(t, J=4.7 Hz, 1H), 2.39(s, 3H) | 168.0, 158.2, 157.4, 132.4, 131.4, 127.4, 125.6, 120.2, 118.1, 116.4, 115.7, 115.5, 21.0 |
| 2l | 8.81—8.55(m, 3H), 8.34(s, 1H), 7.42(d, J=7.9 Hz, 1H), 7.09(d, J=7.8 Hz, 1H), 6.98(t, J=4.7 Hz, 1H), 2.53(s, 3H), 2.38(s, 3H) | 168.2, 158.1, 157.8, 134.7, 133.6, 133.2, 123.8, 121.9, 117.1, 116.5, 115.9, 113.9, 22.2, 21.1 |
| 2m | 9.50(s, 1H), 8.72(d,J=4.6 Hz, 2H), 8.58(s, 1H), 7.95(d, J=8.3 Hz, 1H), 7.59(d, J=8.3 Hz, 1H), 7.08(t, J=4.6 Hz, 1H), 3.98(s, 3H), 2.41(s, 3H) | 168.1, 168.0, 167.9, 158.3, 157.4, 133.1, 132.1, 127.3, 126.3, 123.2, 118.6, 117.7, 117.2, 116.6, 52.2, 29.7, 21.0 |
| 2n | 8.66(d,J=4.6 Hz, 2H), 8.57(d, J=11.0 Hz, 1H), 8.39(s, 1H), 7.52—7.38(m, 1H), 7.02(dd, J=11.8, 6.6 Hz, 2H), 2.39(s, 3H) | 168.0, 162.3, 158.1, 157.5, 133.2, 132.8, 132.7, 120.5, 118.2, 118.1, 116.3, 114.7, 114.7, 110.7, 110.5, 103.9, 103.6 |
| 2o | 8.86(s, 1H), 8.66(d,J=4.5 Hz, 2H), 8.40(s, 1H), 7.45(d, J=8.3 Hz, 1H), 7.24(t, J=8.2 Hz, 1H), 7.04(t, J=4.4 Hz, 1H), 2.38(s, 3H) | 168.0, 158.1, 157.4, 133.2, 132.9, 130.5, 122.7, 122.5, 118.3, 116.6, 116.4, 115.0, 21.0 |
| 2p | 9.03(s, 1H), 8.68(t,J=3.6 Hz, 2H), 8.41(s, 1H), 7.39(q, J=8.4 Hz, 2H), 7.14—6.96(m, 1H), 2.39(s, 3H) | 168.1, 168.0, 158.2, 157.4, 133.2, 125.4, 122.9, 119.5, 118.6, 118.4, 116.5, 115.0, 21.0 |
| 2q | 9.00(dd,J=9.2, 2.5 Hz, 1H), 8.70—8.73(m, 2H), 8.59(d, J=8.8 Hz, 2H), 7.94(d, J=8.0 Hz, 1H), 7.75(d, J=9.2 Hz, 1H), 7.62—7.56(m, 1H), 7.52—7.46(m, 1H), 7.06(s, 1H), 2.53(s, 3H) | 168.0, 158.2, 157.8, 135.4, 130.3, 129.9, 128.4, 127.0, 126.1, 125.1, 124.3, 123.7, 117.1, 116.7, 116.6, 113.8, 21.6 |
| 2r | 8.77(d,J=8.4 Hz, 1H), 8.47(s, 2H), 8.39(s, 1H), 7.55(d, J=7.8 Hz, 1H), 7.36(t, J=7.8 Hz, 1H), 7.24(t, J=7.5 Hz, 1H), 2.39(s, 3H), 2.27(s, 3H) | 168.2, 158.0, 156.1, 133.1, 132.7, 125.1, 124.4, 123.8, 121.9, 117.4, 116.2, 114.7, 21.0, 15.0 |
| 2s | 8.89—8.81(m, 3H), 8.47(s, 1H), 7.57(d, J=6.3 Hz, 3H), 7.50(t, J=7.5 Hz, 2H), 7.46—7.36(m, 2H), 7.27(t, J=8.1 Hz, 1H), 2.40(s, 3H) | 168.1, 156.8, 156.0, 134.4, 133.6, 132.8, 129.4, 129.0, 128.4, 126.5, 124.7, 124.0, 122.3, 117.5, 116.4, 114.6, 21.0 |
| 2t | 8.73—8.60(m, 3H), 8.34(s, 1H), 7.55(d, J=7.8 Hz, 1H), 7.36(t, J=7.8 Hz, 1H), 7.26(t, J=6.3 Hz, 1H), 2.39(s, 3H) | 168.0, 158.5, 155.9, 133.9, 132.7, 124.9, 124.1, 122.5, 117.6, 116.4, 114.4, 113.0, 21.1 |
Table 2 1H NMR and 13C NMR data of compounds 2a—2t
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
|---|---|---|
| 2a | 8.81(d,J=8.4 Hz, 1H), 8.67(d, J=4.8 Hz, 2H), 8.43(s, 1H), 7.56(d, J=7.8 Hz, 1H), 7.37(t, J=7.5 Hz, 1H), 7.29—7.25(m, 1H), 7.02(t, J=4.8 Hz, 1H), 2.40(s, 3H) | 168.2, 158.1, 157.8, 133.5, 132.8, 124.6, 124.0, 122.2, 117.5, 116.4, 116.1, 114.6, 21.1 |
| 2b | 8.73—8.59(m, 3H), 8.36(s, 1H), 7.24(dd, J=10.3, 5.1 Hz, 1H), 7.02—6.92(m, 2H), 2.63(s, 3H), 2.37(s, 3H) | 168.7, 158.1, 157.7, 134.3, 133.2, 129.4, 124.5, 123.9, 122.7, 116.0, 114.8, 114.1, 29.7, 21.3, 18.8 |
| 2c | 8.66(d,J=4.8 Hz, 2H), 8.44(d, J=8.4 Hz, 1H), 8.06(s, 1H), 7.54—7.48(m, 2H), 7.40(dd, J=9.7, 5.6, 1.4 Hz, 3H), 7.25(d, J=4.6 Hz, 1H), 7.02(t, J=4.8 Hz, 1H), 6.74(d, J=7.9 Hz, 1H), 5.11(s, 2H), 1.91(s, 3H) | 169.8, 158.1, 157.7, 152.0, 136.9, 135.1, 133.2, 128.5, 128.4, 128.2, 125.4, 116.2, 114.5, 114.2, 110.0, 104.1, 70.4, 20.4 |
| 2d | 8.79—8.72(m, 1H), 8.66—8.61(m, 2H), 8.27(d, J=2.0 Hz, 1H), 7.20(t, J=7.7 Hz, 2H), 7.02(t, J=4.8, 3.4 Hz, 1H), 2.39(s, 3H) | 169.5, 158.1, 157.2, 134.6, 132.2, 125.0, 124.2, 123.3, 121.4, 116.9, 116.6, 115.3, 21.1 |
| 2e | 9.12(d,J=8.5 Hz, 1H), 8.66(d, J=4.7 Hz, 2H), 8.31(s, 1H), 7.82(d, J=7.5 Hz, 1H), 7.37(t, J=8.0 Hz, 1H), 7.05(t, J=4.6 Hz, 1H), 3.91(d, J=13.3 Hz, 3H), 2.41(s, 3H) | 170.1, 167.2, 158.2, 157.3, 134.5, 132.8, 125.4, 123.5, 123.0, 122.5, 120.7, 118.7, 116.7, 52.2, 21.0 |
| 2f | 8.69—8.59(m, 3H), 8.36(s, 1H), 7.33(s, 1H), 7.18(d, J=8.5 Hz, 1H), 6.97(t, J=4.2 Hz, 1H), 2.47(s, 3H), 2.39(s, 3H) | 168.2, 158.1, 157.7, 133.3, 131.7, 131.2, 126.1, 124.2, 117.2, 116.2, 115.9, 114.6, 21.4, 21.1 |
| 2g | 8.66(dd,J=22.8, 6.8 Hz, 3H), 8.39(s, 1H), 6.98(d, J=8.2 Hz, 3H), 3.89(s, 3H), 2.40(s, 3H) | 168.1, 158.1, 157.6, 155.6, 133.4, 127.8, 124.7, 117.5, 115.8, 115.1, 114.0, 99.4, 55.7, 21.1 |
| 2h | 8.70(d,J=8.7 Hz, 1H), 8.63(d, J=4.7 Hz, 2H), 8.40(s, 1H), 7.49(d, J=7.5 Hz, 2H), 7.40(t, J=7.3 Hz, 2H), 7.36—7.30(m, 1H), 7.06(d, J=9.8 Hz, 2H), 6.98(t, J=4.6 Hz, 1H), 5.13(s, 2H), 2.39(s, 3H), 5.13(s, 2H), 2.39(s, 3H) | 168.1, 158.1, 157.6, 154.8, 137.3, 133.4, 128.6, 127.9, 127.7, 124.7, 117.6, 115.9, 115.2, 114.5, 101.0, 70.6, 21.1 |
| 2i | 8.83—8.76(m, 1H), 8.67(d, J=4.8 Hz, 2H), 8.46(s, 1H), 7.38—7.29(m, 2H), 7.20(d, J=2.4 Hz, 1H), 7.14—6.99(m, 5H), 2.35(s, 3H) | 168.1, 158.6, 158.1, 157.6, 152.0, 133.3, 129.7, 129.5, 125.0, 122.5, 117.8, 117.6, 116.1, 115.7, 107.9, 21.0 |
| 2j | 8.84—8.61(m, 3H), 8.44(s, 1H), 7.52(s, 1H), 7.36—7.24(m, 1H), 7.05(s, 1H), 2.39(s, 3H) | 168.0, 158.2, 157.5, 132.6, 131.1, 127.9, 125.1, 124.8, 117.7, 117.1, 116.4, 115.9, 21.0 |
| 2k | 8.67(t,J=7.2 Hz, 3H), 8.42(s, 1H), 7.68(s, 1H), 7.43(d, J=8.9 Hz, 1H), 7.04(t, J=4.7 Hz, 1H), 2.39(s, 3H) | 168.0, 158.2, 157.4, 132.4, 131.4, 127.4, 125.6, 120.2, 118.1, 116.4, 115.7, 115.5, 21.0 |
| 2l | 8.81—8.55(m, 3H), 8.34(s, 1H), 7.42(d, J=7.9 Hz, 1H), 7.09(d, J=7.8 Hz, 1H), 6.98(t, J=4.7 Hz, 1H), 2.53(s, 3H), 2.38(s, 3H) | 168.2, 158.1, 157.8, 134.7, 133.6, 133.2, 123.8, 121.9, 117.1, 116.5, 115.9, 113.9, 22.2, 21.1 |
| 2m | 9.50(s, 1H), 8.72(d,J=4.6 Hz, 2H), 8.58(s, 1H), 7.95(d, J=8.3 Hz, 1H), 7.59(d, J=8.3 Hz, 1H), 7.08(t, J=4.6 Hz, 1H), 3.98(s, 3H), 2.41(s, 3H) | 168.1, 168.0, 167.9, 158.3, 157.4, 133.1, 132.1, 127.3, 126.3, 123.2, 118.6, 117.7, 117.2, 116.6, 52.2, 29.7, 21.0 |
| 2n | 8.66(d,J=4.6 Hz, 2H), 8.57(d, J=11.0 Hz, 1H), 8.39(s, 1H), 7.52—7.38(m, 1H), 7.02(dd, J=11.8, 6.6 Hz, 2H), 2.39(s, 3H) | 168.0, 162.3, 158.1, 157.5, 133.2, 132.8, 132.7, 120.5, 118.2, 118.1, 116.3, 114.7, 114.7, 110.7, 110.5, 103.9, 103.6 |
| 2o | 8.86(s, 1H), 8.66(d,J=4.5 Hz, 2H), 8.40(s, 1H), 7.45(d, J=8.3 Hz, 1H), 7.24(t, J=8.2 Hz, 1H), 7.04(t, J=4.4 Hz, 1H), 2.38(s, 3H) | 168.0, 158.1, 157.4, 133.2, 132.9, 130.5, 122.7, 122.5, 118.3, 116.6, 116.4, 115.0, 21.0 |
| 2p | 9.03(s, 1H), 8.68(t,J=3.6 Hz, 2H), 8.41(s, 1H), 7.39(q, J=8.4 Hz, 2H), 7.14—6.96(m, 1H), 2.39(s, 3H) | 168.1, 168.0, 158.2, 157.4, 133.2, 125.4, 122.9, 119.5, 118.6, 118.4, 116.5, 115.0, 21.0 |
| 2q | 9.00(dd,J=9.2, 2.5 Hz, 1H), 8.70—8.73(m, 2H), 8.59(d, J=8.8 Hz, 2H), 7.94(d, J=8.0 Hz, 1H), 7.75(d, J=9.2 Hz, 1H), 7.62—7.56(m, 1H), 7.52—7.46(m, 1H), 7.06(s, 1H), 2.53(s, 3H) | 168.0, 158.2, 157.8, 135.4, 130.3, 129.9, 128.4, 127.0, 126.1, 125.1, 124.3, 123.7, 117.1, 116.7, 116.6, 113.8, 21.6 |
| 2r | 8.77(d,J=8.4 Hz, 1H), 8.47(s, 2H), 8.39(s, 1H), 7.55(d, J=7.8 Hz, 1H), 7.36(t, J=7.8 Hz, 1H), 7.24(t, J=7.5 Hz, 1H), 2.39(s, 3H), 2.27(s, 3H) | 168.2, 158.0, 156.1, 133.1, 132.7, 125.1, 124.4, 123.8, 121.9, 117.4, 116.2, 114.7, 21.0, 15.0 |
| 2s | 8.89—8.81(m, 3H), 8.47(s, 1H), 7.57(d, J=6.3 Hz, 3H), 7.50(t, J=7.5 Hz, 2H), 7.46—7.36(m, 2H), 7.27(t, J=8.1 Hz, 1H), 2.40(s, 3H) | 168.1, 156.8, 156.0, 134.4, 133.6, 132.8, 129.4, 129.0, 128.4, 126.5, 124.7, 124.0, 122.3, 117.5, 116.4, 114.6, 21.0 |
| 2t | 8.73—8.60(m, 3H), 8.34(s, 1H), 7.55(d, J=7.8 Hz, 1H), 7.36(t, J=7.8 Hz, 1H), 7.26(t, J=6.3 Hz, 1H), 2.39(s, 3H) | 168.0, 158.5, 155.9, 133.9, 132.7, 124.9, 124.1, 122.5, 117.6, 116.4, 114.4, 113.0, 21.1 |
| Entry | R | T/℃ | Molar fraction(%) | Yieldb(%) | Entry | R | T/℃ | Molar fraction(%) | Yieldb(%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | H | 50 | 150 | 0 | 10 | 2-Pyrimidyl | 25 | 150 | 0 |
| 2 | Me | 50 | 150 | 0 | 11 | 2-Pyrimidyl | 40 | 150 | 45 |
| 3 | Bn | 50 | 150 | 0 | 12 | 2-Pyrimidyl | 60 | 150 | 85 |
| 4 | Ph | 50 | 150 | 40 | 13 | 2-Pyrimidyl | 70 | 150 | 83 |
| 5 | 2-Py | 50 | 150 | 65 | 14 | 2-Pyrimidyl | 80 | 150 | 80 |
| 6 | 2-Pyrimidyl | 50 | 150 | 78 | 15 | 2-Pyrimidyl | 60 | 110 | 83 |
| 7 | Ac | 50 | 150 | 0 | 16 | 2-Pyrimidyl | 60 | 120 | 85 |
| 8 | Boc | 50 | 150 | 0 | 17 | 2-Pyrimidyl | 60 | 200 | 80 |
| 9 | PhSO2 | 50 | 150 | 0 | 18 | 2-Pyrimidyl | 60 | 250 | 76 |
Table 3 Optimization of the reaction conditionsa
| Entry | R | T/℃ | Molar fraction(%) | Yieldb(%) | Entry | R | T/℃ | Molar fraction(%) | Yieldb(%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | H | 50 | 150 | 0 | 10 | 2-Pyrimidyl | 25 | 150 | 0 |
| 2 | Me | 50 | 150 | 0 | 11 | 2-Pyrimidyl | 40 | 150 | 45 |
| 3 | Bn | 50 | 150 | 0 | 12 | 2-Pyrimidyl | 60 | 150 | 85 |
| 4 | Ph | 50 | 150 | 40 | 13 | 2-Pyrimidyl | 70 | 150 | 83 |
| 5 | 2-Py | 50 | 150 | 65 | 14 | 2-Pyrimidyl | 80 | 150 | 80 |
| 6 | 2-Pyrimidyl | 50 | 150 | 78 | 15 | 2-Pyrimidyl | 60 | 110 | 83 |
| 7 | Ac | 50 | 150 | 0 | 16 | 2-Pyrimidyl | 60 | 120 | 85 |
| 8 | Boc | 50 | 150 | 0 | 17 | 2-Pyrimidyl | 60 | 200 | 80 |
| 9 | PhSO2 | 50 | 150 | 0 | 18 | 2-Pyrimidyl | 60 | 250 | 76 |
| Entry | Product | R1 | R2 | Yield*(%) | Entry | Product | R1 | R2 | Yield*(%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2a | H | H | 85 | 11 | 2k | 5-Br | H | 85 |
| 2 | 2b | 4-Me | H | 76 | 12 | 2l | 6-Me | H | 83 |
| 3 | 2c | 4-OBn | H | 63 | 13 | 2m | 6-CO2Me | H | 63 |
| 4 | 2d | 4-Cl | H | 73 | 14 | 2n | 6-F | H | 77 |
| 5 | 2e | 4-CO2Me | H | 75 | 15 | 2o | 6-Cl | H | 70 |
| 6 | 2f | 5-Me | H | 78 | 16 | 2p | 6-Br | H | 80 |
| 7 | 2g | 5-OMe | H | 80 | 17 | 2q | 5,6-Ph | H | 45 |
| 8 | 2h | 5-OBn | H | 65 | 18 | 2r | H | Me | 84 |
| 9 | 2i | 5-OPh | H | 86 | 19 | 2s | H | Ph | 81 |
| 10 | 2j | 5-Cl | H | 83 | 20 | 2t | H | Br | 72 |
Table 4 Substrate scope of the C3—H acetoxylation of indoles
| Entry | Product | R1 | R2 | Yield*(%) | Entry | Product | R1 | R2 | Yield*(%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2a | H | H | 85 | 11 | 2k | 5-Br | H | 85 |
| 2 | 2b | 4-Me | H | 76 | 12 | 2l | 6-Me | H | 83 |
| 3 | 2c | 4-OBn | H | 63 | 13 | 2m | 6-CO2Me | H | 63 |
| 4 | 2d | 4-Cl | H | 73 | 14 | 2n | 6-F | H | 77 |
| 5 | 2e | 4-CO2Me | H | 75 | 15 | 2o | 6-Cl | H | 70 |
| 6 | 2f | 5-Me | H | 78 | 16 | 2p | 6-Br | H | 80 |
| 7 | 2g | 5-OMe | H | 80 | 17 | 2q | 5,6-Ph | H | 45 |
| 8 | 2h | 5-OBn | H | 65 | 18 | 2r | H | Me | 84 |
| 9 | 2i | 5-OPh | H | 86 | 19 | 2s | H | Ph | 81 |
| 10 | 2j | 5-Cl | H | 83 | 20 | 2t | H | Br | 72 |
| [1] | Cacchi S., Fabrizi G., Chem. Rev., 2005, 105(7), 2873—2920 |
| [2] | Kang C. M., Zhao X. H., Yu Y. Q., Lü Y. T., Chem. J. Chinese Universities, 2014, 35(3), 550—554 |
| (康从民, 赵绪浩, 于玉琪, 吕英涛. 高等学校化学学报, 2014, 35(3), 550—554) | |
| [3] | Zhang J. H., Lü Y., Jia H. L., Song Y. Y., Sun X. X., Chai D. X., Wang L. Y., Chem. J. Chinese Universities, 2015, 36(10), 1924—1932 |
| (张江华, 吕英, 贾红亮, 宋银银, 孙晓霞, 柴敦宵, 王兰英. 高等学校化学学报, 2015, 36(10), 1924—1932) | |
| [4] | Cheng X., Li X. L., Wan W. L., Hao W. M., Hai L., Wu Y., Chem. Res. Chinese Universities, 2015, 31(1), 53—59 |
| [5] | Li X. L., Qiu R., Wan W. L., Cheng X., He Y., Hai L., Wu Y., Chem. Res. Chinese Universities, 2015, 31(4), 539—542 |
| [6] | Guyen B., Schultes C. M., Hazel P., Mann J., Neidle S., Org. Biomol. Chem., 2004, 2(7), 981—988 |
| [7] | Arnold R. D., Nutter W. M., Stepp W. L., J. Org. Chem., 1959, 24(1), 117—118 |
| [8] | Bandini M., Eichholzer A., Angew. Chem. Int. Ed., 2009, 48(51), 9608—9644 |
| [9] | Chen J. R., Li C. F., An X. L., Zhang J. J., Zhu X.Y., Xiao W. J., Angew. Chem. Int. Ed., 2008, 47(13), 2489—2492 |
| [10] | An X. L., Chen J. R., Li C. F., Zhang F. G., Zou Y. Q., Guo Y. C., Xiao W. J., Chem. Asian J., 2010, 5(10), 2258—2265 |
| [11] | Cho S. H., Kim J. Y., Kwak J., Chang S., Chem. Soc. Rev., 2011, 40(10), 5068—5083 |
| [12] | Stuart D. R., Fagnou K., Science, 2007, 316(5), 1172—1175 |
| [13] | Liu H. H., Wang Y., Deng G., Yang L., Adv. Synth. Catal., 2013, 355(17), 3369—3374 |
| [14] | Li Y. X., Wang H. X., Ali S., Xia X. F., Liang Y. M., Chem. Commun., 2012, 48(17), 2343—2345 |
| [15] | Liu Q., Li G., Yi H., Wu P., Liu J., Lei A., Chem. Eur. J., 2011, 17(8), 2353—2357 |
| [16] | Sun C. L., Shi Z. J., Chem. Rev., 2014, 114(18), 9219—9280 |
| [17] | Allais C., Grassot J. M., Rodriguez J., Constantieux T., Chem. Rev., 2014, 114(21), 10829—10868 |
| [18] | Zhdankin V. V., Stang P. J., Chem. Rev., 2002, 102(7), 2523—2584 |
| [19] | Wirth T., Angew. Chem. Int. Ed., 2005, 44(24), 3656—3665 |
| [20] | Cho S. H., Yoon J., Chang S., J. Am. Chem. Soc., 2011, 133(15), 5996—6005 |
| [21] | Liu K., Wen P., Liu J., Huang G., Synthesis, 2010, 21, 3623—3626 |
| [22] | Ackermann L., Dell’Acqua M., Fenner S., Vicente R., Sandmann R., Org. Lett., 2011, 13(9), 2358—2360 |
| [23] | Wen J., Zhang R. Y., Chen S. Y., Zhang J., Yu X. Q., J. Org. Chem., 2012, 77(1), 766—771 |
| [24] | Wang L., Qu X., Li Z., Peng W. M., Tetrahedron Lett., 2015, 56(24), 3754—3757 |
| [25] | Wang L., Qu X., Li Z., Peng W. M., Chin. J. Org. Chem., 2015, 35(3), 688—697 |
| (王亮, 瞿星, 李站, 彭望明. 有机化学, 2015, 35(3), 688—697) |
| [1] | 黄秋红, 李文军, 李鑫. 有机催化靛红衍生酮亚胺与噁唑酮的不对称Mannich型加成反应[J]. 高等学校化学学报, 2022, 43(8): 20220131. |
| [2] | 金睿明, 穆晓清, 徐岩. 生物-化学法合成黑色素前体5, 6-二羟基吲哚[J]. 高等学校化学学报, 2022, 43(8): 20220134. |
| [3] | 穆宏文, 吴昊, 高莹莹, 金瑛, 王黎明. 有机催化吲哚碳环上的不对称Friedel⁃Crafts烷基化反应[J]. 高等学校化学学报, 2022, 43(2): 20210571. |
| [4] | 刘苗, 刘瑞波, 刘巴蒂, 钱鹰. 溶酶体靶向吲哚氟硼二吡咯光敏剂的合成、 双光子荧光成像及光动力治疗[J]. 高等学校化学学报, 2022, 43(10): 20220326. |
| [5] | 李鹏杰, 周春妮, 王泽田, 郑子昂, 张玉敏, 王亮, 肖标. 铑催化吲哚与乙烯基三乙氧基硅烷的C—H烯基化反应[J]. 高等学校化学学报, 2021, 42(8): 2450. |
| [6] | 田胜侨, 韦美菊. Rh(Ⅱ)催化吲哚衍生物[3+3]环化机理及产物性质分析[J]. 高等学校化学学报, 2021, 42(6): 1899. |
| [7] | 南江, 陈璞, 马养民. 酸促进2-乙烯基苯胺与重氮的[5+1]环化合成2-芳基喹啉[J]. 高等学校化学学报, 2020, 41(11): 2457. |
| [8] | 马静雨, 刘双磊, 张振国, 金俊阳, 贾振华. B(C6F5)3催化合成二吲哚甲烷类化合物的研究[J]. 高等学校化学学报, 2020, 41(10): 2225. |
| [9] | 苏秋铭, 原安莹, 邝福儿, 陈志强, 白呈超, 辛伟贤. CM⁃Phos配体在钯催化交叉偶联反应中的应用[J]. 高等学校化学学报, 2020, 41(10): 2185. |
| [10] | 刘天伟, 张苏韬, 何江华, 张越涛. B(C6F5)3催化吲哚与苯乙炔的区域选择性加成[J]. 高等学校化学学报, 2019, 40(4): 719. |
| [11] | 李冰, 王学敏, 白凤英, 刘淑清. 稀土氮杂环配合物的合成、 结构及抑菌活性[J]. 高等学校化学学报, 2019, 40(4): 632. |
| [12] | 张莹莹,黄译文,赵冰,王丽艳,宋波. Cr 3+比色荧光探针的合成及细胞成像应用[J]. 高等学校化学学报, 2019, 40(12): 2486. |
| [13] | 谷耀华 薛屏. 离子液体再生纤维素微球共固定漆酶-介体体系对吲哚降解的研究[J]. 高等学校化学学报, 2018, 39(8): 1846. |
| [14] | 郭明, 张新鸽, 曾楚楚, 殷欣欣. 基于Diels-Alder反应的智能分子印迹聚合物制备及性能表征[J]. 高等学校化学学报, 2018, 39(3): 566. |
| [15] | 刘笑宇, 徐议, 唐良富. 3-烷基膦酸酯基取代的异吲哚啉酮衍生物的合成及生物活性[J]. 高等学校化学学报, 2018, 39(11): 2433. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||