

高等学校化学学报 ›› 2023, Vol. 44 ›› Issue (12): 20230337.doi: 10.7503/cjcu20230337
王晓斌1,2,3( ), 王瑞颖1, 董雪1, 严莉莉1, 张娟2, 顾一飞1,2, 程青芳1,2, 薛伟3(
), 王瑞颖1, 董雪1, 严莉莉1, 张娟2, 顾一飞1,2, 程青芳1,2, 薛伟3( )
)
收稿日期:2023-07-23
出版日期:2023-12-10
发布日期:2023-10-08
通讯作者:
王晓斌
E-mail:xb_wang@jou.edu.cn;wxue@gzu.edu.cn
作者简介:薛 伟, 男, 博士, 教授, 主要从事天然仿生型农药分子创制方面的研究. E-mail: wxue@gzu.edu.cn
基金资助:
WANG Xiaobin1,2,3( ), WANG Ruiying1, DONG Xue1, YAN Lili1, ZHANG Juan2, GU Yifei1,2, CHENG Qingfang1,2, XUE Wei3(
), WANG Ruiying1, DONG Xue1, YAN Lili1, ZHANG Juan2, GU Yifei1,2, CHENG Qingfang1,2, XUE Wei3( )
)
Received:2023-07-23
Online:2023-12-10
Published:2023-10-08
Contact:
WANG Xiaobin
E-mail:xb_wang@jou.edu.cn;wxue@gzu.edu.cn
Supported by:摘要:
为了开发新型抗肿瘤药物候选分子, 将香豆素单元有机融入1,4-戊二烯-3-酮分子骨架中, 设计合成了16个结构新颖的单羰基姜黄素衍生物. 在确证目标分子结构后, 采用甲基四氮唑盐(MTT)比色法测试了其对胃癌SGC7901细胞和肝癌HepG2细胞体外增殖的抑制活性. 生物活性测试结果表明, 绝大多数目标分子均能显著抑制SGC7901和HepG2细胞的体外增殖. 其中, 化合物4c和4j对SGC7901细胞的半数抑制浓度(IC50)为0.22和0.27 µmol/L, 其活性显著优于对照药剂表柔比星(1.23 µmol/L). 同时, 化合物4l对HepG2细胞的IC50 值(0.47 µmol/L)也显著优于表柔比星(2.30 µmol/L). 细胞形态学研究结果进一步证实, 含香豆素结构1,4-戊二烯-3-酮衍生物能显著抑制多种肿瘤细胞的体外增殖, 可作为高效抗肿瘤药物候选分子进行深度开发.
中图分类号:
TrendMD:
王晓斌, 王瑞颖, 董雪, 严莉莉, 张娟, 顾一飞, 程青芳, 薛伟. 含香豆素结构的1,4-戊二烯-3-酮类衍生物的合成及生物活性. 高等学校化学学报, 2023, 44(12): 20230337.
WANG Xiaobin, WANG Ruiying, DONG Xue, YAN Lili, ZHANG Juan, GU Yifei, CHENG Qingfang, XUE Wei. Synthesis and Bioactivities of Penta-1,4-dien-3-one Derivatives Containing a Coumarin Moiety. Chem. J. Chinese Universities, 2023, 44(12): 20230337.
| Compound | X | Ar | Appearance | Yield(%) | m. p./℃ | HRMS[M+H]+ | |
|---|---|---|---|---|---|---|---|
| Calculated | Found | ||||||
| 1a | 2⁃O⁃ | — | Yellow solid | 77.2 | 134—136 | — | — |
| 1b | 4⁃O⁃ | — | Yellow solid | 75.1 | 96—98 | — | — |
| 2a | 4⁃O⁃ | Pyrid⁃2⁃yl | Yellow solid | 75.0 | 153—155 | — | — |
| 2b | 4⁃O⁃ | Pyrid⁃3⁃yl | Yellow solid | 75.3 | 173—175 | — | — |
| 2c | 2⁃O⁃ | 2,4⁃di⁃OMePh | Yellow solid | 57.8 | 164—166 | — | — |
| 2d | 2⁃O⁃ | Ph | Yellow solid | 68.6 | 183—185 | — | — |
| 2e | 4⁃O⁃ | 2⁃OMePh | Yellow solid | 74.6 | 173—175 | — | — |
| 2f | 4⁃O⁃ | 4⁃ClPh | Yellow solid | 76.9 | 217—219 | — | — |
| 2g | 4⁃O⁃ | 3,4⁃di⁃OMePh | Yellow solid | 62.8 | 158—160 | — | — |
| 2h | 4⁃O⁃ | Fural⁃2⁃yl | Yellow solid | 69.1 | 162—164 | — | — |
| 2i | 2⁃O⁃ | Thiophen⁃2⁃yl | Yellow solid | 72.0 | 153—155 | — | — |
| 2j | 2⁃O⁃ | 2⁃OMePh | Yellow solid | 78.5 | 161—163 | — | — |
| 2k | 2⁃O⁃ | 4⁃FPh | Yellow solid | 80.2 | 121—123 | — | — |
| 2l | 2⁃O⁃ | 4⁃OMePh | Yellow solid | 59.4 | 172—174 | — | — |
| 2m | 2⁃O⁃ | Pyrid⁃2⁃yl | Yellow solid | 66.3 | 158—160 | — | — |
| 2n | 4⁃O⁃ | 4⁃FPh | Yellow solid | 69.2 | 134—136 | — | — |
| 2o | 4⁃O⁃ | Thiophen⁃2⁃yl | Yellow solid | 68.4 | 207—209 | — | — |
| 2p | 4⁃O⁃ | Ph | Yellow solid | 63.5 | 170—171 | — | — |
| 3 | — | — | White solid | 56.2 | 110—112 | — | — |
| 4a | 4⁃O⁃ | Pyrid⁃2⁃yl | Yellow solid | 61.5 | 188—190 | 396.1230 | 396.1221 |
| 4b | 4⁃O⁃ | Pyrid⁃3⁃yl | Yellow solid | 61.3 | 211—213 | 396.1230 | 396.1220 |
| 4c | 2⁃O⁃ | 2,4⁃di⁃OMePh | Yellow solid | 42.7 | 112—114 | 455.1489 | 455.1484 |
| 4d | 2⁃O⁃ | Ph | Yellow solid | 73.7 | 163—165 | 395.1278 | 395.1276 |
| 4e | 4⁃O⁃ | 2⁃OMePh | Yellow solid | 69.7 | 229—230 | 325.1384 | 425.1374 |
| 4f | 4⁃O⁃ | 4⁃ClPh | Yellow solid | 34.6 | 241—243 | 429.0888 | 429.0883 |
| 4g | 4⁃O⁃ | 3,4⁃di⁃OMePh | Yellow solid | 29.4 | 206—208 | 455.1489 | 455.1483 |
| 4h | 4⁃O⁃ | Fural⁃2⁃yl | Yellow solid | 58.5 | 185—187 | 385.1071 | 385.1063 |
| 4i | 2⁃O⁃ | Thiophen⁃2⁃yl | Yellow solid | 53.0 | 195—197 | 401.0842 | 401.0839 |
| 4j | 2⁃O⁃ | 2⁃OMePh | Yellow solid | 39.5 | 123—125 | 425.1384 | 425.1378 |
| 4k | 2⁃O⁃ | 4⁃FPh | White solid | 58.2 | 187—189 | 413.1184 | 413.1180 |
| 4l | 2⁃O⁃ | 4⁃OMePh | Yellow solid | 54.1 | 224—226 | 425.1384 | 425.1381 |
| 4m | 2⁃O⁃ | Pyrid⁃2⁃yl | Yellow solid | 81.3 | 179—181 | 396.1230 | 396.1228 |
| 4n | 4⁃O⁃ | 4⁃FPh | Yellow solid | 43.2 | 195—197 | 413.1184 | 413.1180 |
| 4o | 4⁃O⁃ | Thiophen⁃2⁃yl | Yellow solid | 65.3 | 162—164 | 401.0842 | 401.0838 |
| 4p | 4⁃O⁃ | Ph | White solid | 71.2 | 203—205 | 395.1278 | 395.1275 |
Table 1 Appearance, yields, melting points and HRMS data of compounds 1—4
| Compound | X | Ar | Appearance | Yield(%) | m. p./℃ | HRMS[M+H]+ | |
|---|---|---|---|---|---|---|---|
| Calculated | Found | ||||||
| 1a | 2⁃O⁃ | — | Yellow solid | 77.2 | 134—136 | — | — |
| 1b | 4⁃O⁃ | — | Yellow solid | 75.1 | 96—98 | — | — |
| 2a | 4⁃O⁃ | Pyrid⁃2⁃yl | Yellow solid | 75.0 | 153—155 | — | — |
| 2b | 4⁃O⁃ | Pyrid⁃3⁃yl | Yellow solid | 75.3 | 173—175 | — | — |
| 2c | 2⁃O⁃ | 2,4⁃di⁃OMePh | Yellow solid | 57.8 | 164—166 | — | — |
| 2d | 2⁃O⁃ | Ph | Yellow solid | 68.6 | 183—185 | — | — |
| 2e | 4⁃O⁃ | 2⁃OMePh | Yellow solid | 74.6 | 173—175 | — | — |
| 2f | 4⁃O⁃ | 4⁃ClPh | Yellow solid | 76.9 | 217—219 | — | — |
| 2g | 4⁃O⁃ | 3,4⁃di⁃OMePh | Yellow solid | 62.8 | 158—160 | — | — |
| 2h | 4⁃O⁃ | Fural⁃2⁃yl | Yellow solid | 69.1 | 162—164 | — | — |
| 2i | 2⁃O⁃ | Thiophen⁃2⁃yl | Yellow solid | 72.0 | 153—155 | — | — |
| 2j | 2⁃O⁃ | 2⁃OMePh | Yellow solid | 78.5 | 161—163 | — | — |
| 2k | 2⁃O⁃ | 4⁃FPh | Yellow solid | 80.2 | 121—123 | — | — |
| 2l | 2⁃O⁃ | 4⁃OMePh | Yellow solid | 59.4 | 172—174 | — | — |
| 2m | 2⁃O⁃ | Pyrid⁃2⁃yl | Yellow solid | 66.3 | 158—160 | — | — |
| 2n | 4⁃O⁃ | 4⁃FPh | Yellow solid | 69.2 | 134—136 | — | — |
| 2o | 4⁃O⁃ | Thiophen⁃2⁃yl | Yellow solid | 68.4 | 207—209 | — | — |
| 2p | 4⁃O⁃ | Ph | Yellow solid | 63.5 | 170—171 | — | — |
| 3 | — | — | White solid | 56.2 | 110—112 | — | — |
| 4a | 4⁃O⁃ | Pyrid⁃2⁃yl | Yellow solid | 61.5 | 188—190 | 396.1230 | 396.1221 |
| 4b | 4⁃O⁃ | Pyrid⁃3⁃yl | Yellow solid | 61.3 | 211—213 | 396.1230 | 396.1220 |
| 4c | 2⁃O⁃ | 2,4⁃di⁃OMePh | Yellow solid | 42.7 | 112—114 | 455.1489 | 455.1484 |
| 4d | 2⁃O⁃ | Ph | Yellow solid | 73.7 | 163—165 | 395.1278 | 395.1276 |
| 4e | 4⁃O⁃ | 2⁃OMePh | Yellow solid | 69.7 | 229—230 | 325.1384 | 425.1374 |
| 4f | 4⁃O⁃ | 4⁃ClPh | Yellow solid | 34.6 | 241—243 | 429.0888 | 429.0883 |
| 4g | 4⁃O⁃ | 3,4⁃di⁃OMePh | Yellow solid | 29.4 | 206—208 | 455.1489 | 455.1483 |
| 4h | 4⁃O⁃ | Fural⁃2⁃yl | Yellow solid | 58.5 | 185—187 | 385.1071 | 385.1063 |
| 4i | 2⁃O⁃ | Thiophen⁃2⁃yl | Yellow solid | 53.0 | 195—197 | 401.0842 | 401.0839 |
| 4j | 2⁃O⁃ | 2⁃OMePh | Yellow solid | 39.5 | 123—125 | 425.1384 | 425.1378 |
| 4k | 2⁃O⁃ | 4⁃FPh | White solid | 58.2 | 187—189 | 413.1184 | 413.1180 |
| 4l | 2⁃O⁃ | 4⁃OMePh | Yellow solid | 54.1 | 224—226 | 425.1384 | 425.1381 |
| 4m | 2⁃O⁃ | Pyrid⁃2⁃yl | Yellow solid | 81.3 | 179—181 | 396.1230 | 396.1228 |
| 4n | 4⁃O⁃ | 4⁃FPh | Yellow solid | 43.2 | 195—197 | 413.1184 | 413.1180 |
| 4o | 4⁃O⁃ | Thiophen⁃2⁃yl | Yellow solid | 65.3 | 162—164 | 401.0842 | 401.0838 |
| 4p | 4⁃O⁃ | Ph | White solid | 71.2 | 203—205 | 395.1278 | 395.1275 |
| Compound | 1H NMR(500 MHz, DMSO⁃d6 or CDCl3) | 13C NMR(125 MHz, DMSO⁃d6 or CDCl3) |
|---|---|---|
| 4a | 8.64(d, J=7.5 Hz, 1H), 8.02—7.95(m, 3H), 7.87(td, J=7.6, 1.7 Hz, 1H), 7.81(d, J=5.9 Hz, 1H), 7.78(d, J=5.6 Hz, 1H), 7.75(d, J=7.8 Hz, 1H), 7.74—7.69(m, 1H), 7.57(d, J=15.8 Hz, 1H), 7.46(d, J=2.6 Hz, 1H), 7.44(dd, J=7.0, 1.5 Hz, 3H), 7.40(ddd, J=9.0, 5.5, 2.7 Hz, 2H), 5.27(s, 1H) | 189.21, 165.90, 161.60, 154.23, 153.63, 153.30, 150.62, 142.49, 142.39, 137.81, 133.98, 133.79, 131.50, 129.22, 126.56, 125.69, 125.35, 125.07, 123.57, 122.38, 117.16, 115.27, 94.02 |
| 4b | 8.93(s, 1H), 8.59(d, J=4.5 Hz, 1H), 8.21(d, J=7.9 Hz, 1H), 8.01(d, J=7.9 Hz, 1H), 7.96(d, J=8.4 Hz, 2H), 7.87(d, J=16.1 Hz, 1H), 7.81(d, J=16.2 Hz, 1H), 7.73(t, J=7.8 Hz, 1H), 7.51—7.41(m, 6H), 7.37(d, J=16.1 Hz, 1H), 5.29(s, 1H) | 188.92, 165.91, 161.60, 154.25, 153.65, 151.55, 150.63, 142.53, 140.09, 135.34, 134.02, 133.82, 131.39, 131.09, 127.74, 126.65, 125.11, 124.57, 123.59, 122.45, 117.18, 115.29, 94.06 |
| 4c | 8.20(d, J=6.5 Hz, 1H), 8.10—8.05(m, 1H), 7.94(dd, J=16.1, 4.7 Hz, 1H), 7.78—7.70(m, 1H), 7.64(d, J=11.4 Hz, 1H), 7.58—7.53(m, 1H), 7.53—7.43(m, 3H), 7.37(dd, J=20.8, 12.4 Hz, 3H), 7.13(dd, J=16.1, 4.7 Hz, 1H), 7.04(dd, J=8.1, 4.5 Hz, 1H), 6.94(dd, J=12.0, 7.3 Hz, 1H), 5.19(d, J=4.9 Hz, 1H), 3.85(s, 3H), 2.89(s Hz, 3H) | 188.02, 165.73, 160.90, 158.77, 153.86, 151.32, 138.26, 134.20, 133.39, 132.26, 132.08, 128.87, 128.56, 177.60, 125.91, 124.53, 123.39, 123.10, 122.73, 120.75, 116.75, 115.07, 111.54, 93.76, 59.75, 55.19 |
| 4d | 8.10(d, J=6.5 Hz, 1H), 7.82(dd, J=7.9, 1.0 Hz, 1H), 7.73(d, J=8.1 Hz, 1H), 7.70(d, J=8.3 Hz, 1H), 7.6—7.63(m, 1H), 7.54—7.49(m, 1H), 7.43—7.36(m, 5H), 7.24(d, J=3.5 Hz, 1H), 7.21(d, J=8.0 Hz, 1H), 7.10—7.02(m, 3H), 6.68(d, J=15.6 Hz, 1H), 5.32(s, 1H) | 187.86, 165.95, 162.39, 153.81, 151.30, 140.12, 136.35, 135.17, 133.22, 132.14, 132.10, 129.23, 128.90, 128.46, 128.07, 128.01, 127.48, 124.53, 124.48, 123.05, 122.61, 117.17, 115.09, 94.29 |
| 4e | 8.06—7.97(m, 2H), 7.95(d, J=5.9 Hz, 2H), 7.80(t, J=10.9 Hz, 2H), 7.75—7.69(m, 1H), 7.43(dt, J=14.7, 8.3 Hz, 5H), 7.36(d, J=16.1 Hz, 1H), 7.30(d, J=16.2 Hz, 1H), 7.08(d, J=8.3 Hz, 1H), 7.00(t, J=7.4 Hz, 1H), 5.27(s, 1H), 3.87(s, 3H) | 189.03, 165.93, 161.62, 158.78, 154.12, 153.65, 141.88, 138.05, 134.01, 133.91, 132.78, 131.35, 129.07, 127.42, 126.05, 125.10, 123.60, 123.42, 122.41, 121.27, 117.18, 115.29, 112.37, 94.00, 56.23 |
| 4f | 8.00(dd, J=7.9, 1.4 Hz, 1H), 7.95(d, J=8.6 Hz, 2H), 7.83(t, J=9.3 Hz, 1H), 7.78(dd, J=16.7, 7.5 Hz, 3H), 7.75—7.70(m, 1H), 7.50(d, J=8.5 Hz, 2H), 7.44(ddd, J=14.1, 8.0, 3.3 Hz, 4H), 7.37(d, J=8.1 Hz, 1H), 7.34(d, J=8.2 Hz, 1H), 5.28(s, 1H) | 188.98, 165.91, 161.61, 154.18, 153.64, 142.22, 142.06, 135.58, 134.21, 134.01, 133.86, 131.36, 130.78, 129.60, 126.79, 126.68, 125.10, 123.58, 122.43, 117.18, 115.28, 94.01 |
| 4g | 8.01(dd, J=7.9, 1.2 Hz, 1H), 7.96(d, J=8.7 Hz, 2H), 7.78(d, J=8.9 Hz, 1H), 7.76—7.70(m, 2H), 7.45(dd, J=10.8, 8.5 Hz, 4H), 7.40(dd, J=14.8, 4.6 Hz, 2H), 7.31(dd, J=8.4, 1.8 Hz, 1H), 7.18(d, J=16.0 Hz, 1H), 7.01(d, J=8.4 Hz, 1H), 5.28(s, 1H), 3.81(s, 3H), 3.78(s, 3H) | 188.81, 165.95, 161.62, 154.03, 153.64, 151.72, 149.51, 144.09, 141.30, 134.01, 131.26, 127.97, 126.56, 125.10, 124.48, 123.92, 123.58, 122.40, 117.18, 115.29, 112.15, 110.99, 93.97, 56.12 |
| 4h | 8.01(dd, J=7.5, 2.4 Hz, 1H), 7.75—7.68(m, 3H), 7.62(dd, J=11.4, 4.2 Hz, 1H), 7.56—7.49(m, 2H), 7.37(td, J=7.9, 4.0 Hz, 2H), 7.23(dd, J=8.1, 3.3 Hz, 2H), 7.00(dd, J=15.7, 3.5 Hz, 2H), 6.72(d, J=3.4 Hz, 1H), 6.52(s, 1H), 5.46(d, J=3.6 Hz, 1H) | 188.28, 166.01, 162.47, 153.87, 153.76, 151.51, 145.22, 141.30, 133.69, 133.11, 130.46, 129.85, 126.89, 124.33, 123.14, 122.51, 122.03, 117.03, 116.48, 115.30, 112.85, 93.99 |
| 4i | 8.11(dd, J=7.8, 1.3 Hz, 1H), 7.82(dd, J=7.8, 1.4 Hz, 1H), 7.73(d, J=8.2 Hz, 1H), 7.70(d, J=8.5 Hz, 1H), 7.65(td, J=7.7, 1.6 Hz, 1H), 7.54—7.49(m, 1H), 7.40(dd, J=13.4, 5.8 Hz, 4H), 7.24(d, J=3.5 Hz, 1H), 7.21(dd, J=8.0, 0.9 Hz, 1H), 7.09—7.03(m, 2H), 6.69(d, J=15.6 Hz, 1H), 5.32(s, 1H) | 187.86, 165.95, 162.40, 153.81, 151.30, 140.12, 136.35, 135.17, 133.22, 132.14, 132.10, 129.23, 128.90, 128.46, 128.07, 128.01, 127.48, 124.53, 124.48, 123.05, 122.62, 117.17, 115.10, 94.29 |
| 4j | 8.10(d, J=8.1 Hz, 1H), 7.95(d, J=16.2 Hz, 1H), 7.85(d, J=7.7 Hz, 1H), 7.71(d, J=15.9 Hz, 1H), 7.63(t, J=7.8 Hz, 1H), 7.51(t, J=7.7 Hz, 1H), 7.45(d, J=7.7 Hz, 1H), 7.43—7.32(m, 4H), 7.20(d, J=8.1 Hz, 1H), 7.19—7.14(m, 1H), 6.97(d, J=16.1 Hz, 1H), 6.93(t, J=7.5 Hz, 1H), 6.89(d, J=8.3 Hz, 1H), 5.37—5.30(m, 1H), 3.86(d, J=17.1 Hz, 3H) | 189.11, 166.04, 162.47, 158.72, 153.78, 151.24, 139.46, 134.80, 133.18, 132.05, 131.99, 129.08, 128.69, 128.24, 127.96, 127.47, 126.36, 124.49, 123.53, 123.09, 122.58, 120.84, 117.11, 115.09, 111.24, 94.26, 55.56 |
| 4k | 8.11(d, J=7.2 Hz, 1H), 7.83(d, J=7.6 Hz, 1H), 7.71(d, J=16.0 Hz, 1H), 7.65(t, J=7.7 Hz, 1H), 7.53(dd, J=21.2, 11.8 Hz, 2H), 7.44(dd, J=8.0, 5.0 Hz, 2H), 7.40(dd, J=14.2, 7.6 Hz, 3H), 7.21(d, J=8.0 Hz, 1H), 7.10(t, J=11.3 Hz, 1H), 7.04(t, J=8.5 Hz, 2H), 6.80(d, J=16.0 Hz, 1H), 5.32(s, 1H) | 188.30, 165.94, 164.17(d, J=252.3 Hz), 162.34, 153.81, 151.31, 142.58, 135.42, 133.21, 132.19, 130.85(d, J=2.6 Hz), 130.35(d, J=8.5 Hz), 128.85, 128.00, 127.84, 127.52, 125.28, 124.50, 123.03, 122.64, 117.18, 116.22(d, J=21.8 Hz), 115.08, 94.35 |
| 4l | 8.12(d, J=8.0 Hz, 1H), 7.83(d, J=7.8 Hz, 1H), 7.71(d, J=15.9 Hz, 1H), 7.65(t, J=7.9 Hz, 1H), 7.57(d, J=15.9 Hz, 1H), 7.51(t, J=7.7 Hz, 1H), 7.44—7.38(m, 5H), 7.21(d, J=8.1 Hz, 1H), 7.12(d, J=16.0 Hz, 1H), 6.88(d, J=8.5 Hz, 2H), 6.76(d, J=15.4 Hz, 1H), 5.33(s, 1H), 3.83(d, J=4.2 Hz, 3H) | 188.43, 165.97, 162.41, 161.86, 153.81, 151.26, 143.80, 134.85, 133.18, 131.98, 130.27, 128.87, 128.20, 128.06, 127.47, 127.29, 124.50, 123.50, 123.07, 122.60, 117.17, 115.12, 114.51, 94.31, 55.52 |
| 4m | 8.61(d, J=4.6 Hz, 1H), 8.10(d, J=7.9 Hz, 1H), 7.83(d, J=7.8 Hz, 1H), 7.77(d, J=16.0 Hz, 1H), 7.69(dd, J=8.3, 6.9 Hz, 1H), 7.62(dd, J=20.5, 11.8 Hz, 2H), 7.52(t, J=7.7 Hz, 1H), 7.44—7.36(m, 5H), 7.26(t, J=3.8 Hz, 1H), 7.20(d, J=8.1 Hz, 1H), 7.15(d, J=16.0 Hz, 1H), 5.32(s, 1H) | 188.73, 166.00, 162.39, 153.79, 153.08, 151.41, 150.25, 142.20, 136.92, 135.78, 133.17, 132.27, 128.90, 128.72, 127.98, 127.49, 125.01, 124.54, 124.52, 123.12, 122.59, 117.10, 115.10, 94.37 |
| 4n | 8.01(dd, J=7.9, 1.4 Hz, 1H), 7.78—7.70(m, 4H), 7.65—7.60(m, 3H), 7.40—7.34(m, 2H), 7.24(d, J=8.6 Hz, 2H), 7.14—7.05(m, 3H), 7.01(d, J=15.9 Hz, 1H), 5.46(s, 1H) | 188.46, 166.02, 164.21(d, J=252.1 Hz), 162.48, 153.95, 153.76, 142.52, 141.65, 133.60, 133.14, 131.00(d, J=3.0 Hz), 130.50, 130.44, 126.22, 125.13, 124.35, 123.14, 122.08, 117.05, 116.39, 116.22, 115.28, 94.00 |
| 4o | 8.01(dd, J=7.9, 1.4 Hz, 1H), 7.88(d, J=15.5 Hz, 1H), 7.74—7.70(m, 3H), 7.62(t, J=12.6 Hz, 1H), 7.42(d, J=5.1 Hz, 1H), 7.39—7.34(m, 3H), 7.23(d, J=8.6 Hz, 2H), 7.09(dd, J=5.0, 3.8 Hz, 1H), 7.03(d, J=16.0 Hz, 1H), 6.88(d, J=15.5 Hz, 1H), 5.46(s, 1H) | 188.06, 165.98, 162.41, 153.91, 153.78, 141.33, 140.28, 136.26, 133.67, 133.09, 132.17, 130.46, 129.16, 128.51, 126.45, 124.31, 124.25, 123.13, 122.01, 117.02, 115.31, 94.02 |
| 4p | 8.21(d, J=2.6 Hz, 1H), 8.19(s, 2H), 7.60(d, J=8.4 Hz, 1H), 7.35 8.02(dd, J=7.8, 1.2 Hz, 1H), 7.77(d, J=6.8 Hz, 1H), 7.75—7.72(m, 3H), 7.65—7.60(m, 3H), 7.42(dd, J=6.2, 3.8 Hz, 3H), 7.40—7.34(m, 2H), 7.24(d, J=8.6 Hz, 2H), 7.11(d, J=5.9 Hz, 1H), 7.08(d, J=5.9 Hz, 1H), 5.47(s, 1H) | 188.68, 166.00, 162.44, 153.93, 153.78, 143.83, 141.54, 134.77, 133.68, 133.11, 130.75, 130.48, 129.10, 128.55, 126.24, 125.49, 124.32, 123.14, 122.03, 117.04, 115.31, 94.03 |
Table 2 1H NMR and 13C NMR data of title compounds 4a—4p
| Compound | 1H NMR(500 MHz, DMSO⁃d6 or CDCl3) | 13C NMR(125 MHz, DMSO⁃d6 or CDCl3) |
|---|---|---|
| 4a | 8.64(d, J=7.5 Hz, 1H), 8.02—7.95(m, 3H), 7.87(td, J=7.6, 1.7 Hz, 1H), 7.81(d, J=5.9 Hz, 1H), 7.78(d, J=5.6 Hz, 1H), 7.75(d, J=7.8 Hz, 1H), 7.74—7.69(m, 1H), 7.57(d, J=15.8 Hz, 1H), 7.46(d, J=2.6 Hz, 1H), 7.44(dd, J=7.0, 1.5 Hz, 3H), 7.40(ddd, J=9.0, 5.5, 2.7 Hz, 2H), 5.27(s, 1H) | 189.21, 165.90, 161.60, 154.23, 153.63, 153.30, 150.62, 142.49, 142.39, 137.81, 133.98, 133.79, 131.50, 129.22, 126.56, 125.69, 125.35, 125.07, 123.57, 122.38, 117.16, 115.27, 94.02 |
| 4b | 8.93(s, 1H), 8.59(d, J=4.5 Hz, 1H), 8.21(d, J=7.9 Hz, 1H), 8.01(d, J=7.9 Hz, 1H), 7.96(d, J=8.4 Hz, 2H), 7.87(d, J=16.1 Hz, 1H), 7.81(d, J=16.2 Hz, 1H), 7.73(t, J=7.8 Hz, 1H), 7.51—7.41(m, 6H), 7.37(d, J=16.1 Hz, 1H), 5.29(s, 1H) | 188.92, 165.91, 161.60, 154.25, 153.65, 151.55, 150.63, 142.53, 140.09, 135.34, 134.02, 133.82, 131.39, 131.09, 127.74, 126.65, 125.11, 124.57, 123.59, 122.45, 117.18, 115.29, 94.06 |
| 4c | 8.20(d, J=6.5 Hz, 1H), 8.10—8.05(m, 1H), 7.94(dd, J=16.1, 4.7 Hz, 1H), 7.78—7.70(m, 1H), 7.64(d, J=11.4 Hz, 1H), 7.58—7.53(m, 1H), 7.53—7.43(m, 3H), 7.37(dd, J=20.8, 12.4 Hz, 3H), 7.13(dd, J=16.1, 4.7 Hz, 1H), 7.04(dd, J=8.1, 4.5 Hz, 1H), 6.94(dd, J=12.0, 7.3 Hz, 1H), 5.19(d, J=4.9 Hz, 1H), 3.85(s, 3H), 2.89(s Hz, 3H) | 188.02, 165.73, 160.90, 158.77, 153.86, 151.32, 138.26, 134.20, 133.39, 132.26, 132.08, 128.87, 128.56, 177.60, 125.91, 124.53, 123.39, 123.10, 122.73, 120.75, 116.75, 115.07, 111.54, 93.76, 59.75, 55.19 |
| 4d | 8.10(d, J=6.5 Hz, 1H), 7.82(dd, J=7.9, 1.0 Hz, 1H), 7.73(d, J=8.1 Hz, 1H), 7.70(d, J=8.3 Hz, 1H), 7.6—7.63(m, 1H), 7.54—7.49(m, 1H), 7.43—7.36(m, 5H), 7.24(d, J=3.5 Hz, 1H), 7.21(d, J=8.0 Hz, 1H), 7.10—7.02(m, 3H), 6.68(d, J=15.6 Hz, 1H), 5.32(s, 1H) | 187.86, 165.95, 162.39, 153.81, 151.30, 140.12, 136.35, 135.17, 133.22, 132.14, 132.10, 129.23, 128.90, 128.46, 128.07, 128.01, 127.48, 124.53, 124.48, 123.05, 122.61, 117.17, 115.09, 94.29 |
| 4e | 8.06—7.97(m, 2H), 7.95(d, J=5.9 Hz, 2H), 7.80(t, J=10.9 Hz, 2H), 7.75—7.69(m, 1H), 7.43(dt, J=14.7, 8.3 Hz, 5H), 7.36(d, J=16.1 Hz, 1H), 7.30(d, J=16.2 Hz, 1H), 7.08(d, J=8.3 Hz, 1H), 7.00(t, J=7.4 Hz, 1H), 5.27(s, 1H), 3.87(s, 3H) | 189.03, 165.93, 161.62, 158.78, 154.12, 153.65, 141.88, 138.05, 134.01, 133.91, 132.78, 131.35, 129.07, 127.42, 126.05, 125.10, 123.60, 123.42, 122.41, 121.27, 117.18, 115.29, 112.37, 94.00, 56.23 |
| 4f | 8.00(dd, J=7.9, 1.4 Hz, 1H), 7.95(d, J=8.6 Hz, 2H), 7.83(t, J=9.3 Hz, 1H), 7.78(dd, J=16.7, 7.5 Hz, 3H), 7.75—7.70(m, 1H), 7.50(d, J=8.5 Hz, 2H), 7.44(ddd, J=14.1, 8.0, 3.3 Hz, 4H), 7.37(d, J=8.1 Hz, 1H), 7.34(d, J=8.2 Hz, 1H), 5.28(s, 1H) | 188.98, 165.91, 161.61, 154.18, 153.64, 142.22, 142.06, 135.58, 134.21, 134.01, 133.86, 131.36, 130.78, 129.60, 126.79, 126.68, 125.10, 123.58, 122.43, 117.18, 115.28, 94.01 |
| 4g | 8.01(dd, J=7.9, 1.2 Hz, 1H), 7.96(d, J=8.7 Hz, 2H), 7.78(d, J=8.9 Hz, 1H), 7.76—7.70(m, 2H), 7.45(dd, J=10.8, 8.5 Hz, 4H), 7.40(dd, J=14.8, 4.6 Hz, 2H), 7.31(dd, J=8.4, 1.8 Hz, 1H), 7.18(d, J=16.0 Hz, 1H), 7.01(d, J=8.4 Hz, 1H), 5.28(s, 1H), 3.81(s, 3H), 3.78(s, 3H) | 188.81, 165.95, 161.62, 154.03, 153.64, 151.72, 149.51, 144.09, 141.30, 134.01, 131.26, 127.97, 126.56, 125.10, 124.48, 123.92, 123.58, 122.40, 117.18, 115.29, 112.15, 110.99, 93.97, 56.12 |
| 4h | 8.01(dd, J=7.5, 2.4 Hz, 1H), 7.75—7.68(m, 3H), 7.62(dd, J=11.4, 4.2 Hz, 1H), 7.56—7.49(m, 2H), 7.37(td, J=7.9, 4.0 Hz, 2H), 7.23(dd, J=8.1, 3.3 Hz, 2H), 7.00(dd, J=15.7, 3.5 Hz, 2H), 6.72(d, J=3.4 Hz, 1H), 6.52(s, 1H), 5.46(d, J=3.6 Hz, 1H) | 188.28, 166.01, 162.47, 153.87, 153.76, 151.51, 145.22, 141.30, 133.69, 133.11, 130.46, 129.85, 126.89, 124.33, 123.14, 122.51, 122.03, 117.03, 116.48, 115.30, 112.85, 93.99 |
| 4i | 8.11(dd, J=7.8, 1.3 Hz, 1H), 7.82(dd, J=7.8, 1.4 Hz, 1H), 7.73(d, J=8.2 Hz, 1H), 7.70(d, J=8.5 Hz, 1H), 7.65(td, J=7.7, 1.6 Hz, 1H), 7.54—7.49(m, 1H), 7.40(dd, J=13.4, 5.8 Hz, 4H), 7.24(d, J=3.5 Hz, 1H), 7.21(dd, J=8.0, 0.9 Hz, 1H), 7.09—7.03(m, 2H), 6.69(d, J=15.6 Hz, 1H), 5.32(s, 1H) | 187.86, 165.95, 162.40, 153.81, 151.30, 140.12, 136.35, 135.17, 133.22, 132.14, 132.10, 129.23, 128.90, 128.46, 128.07, 128.01, 127.48, 124.53, 124.48, 123.05, 122.62, 117.17, 115.10, 94.29 |
| 4j | 8.10(d, J=8.1 Hz, 1H), 7.95(d, J=16.2 Hz, 1H), 7.85(d, J=7.7 Hz, 1H), 7.71(d, J=15.9 Hz, 1H), 7.63(t, J=7.8 Hz, 1H), 7.51(t, J=7.7 Hz, 1H), 7.45(d, J=7.7 Hz, 1H), 7.43—7.32(m, 4H), 7.20(d, J=8.1 Hz, 1H), 7.19—7.14(m, 1H), 6.97(d, J=16.1 Hz, 1H), 6.93(t, J=7.5 Hz, 1H), 6.89(d, J=8.3 Hz, 1H), 5.37—5.30(m, 1H), 3.86(d, J=17.1 Hz, 3H) | 189.11, 166.04, 162.47, 158.72, 153.78, 151.24, 139.46, 134.80, 133.18, 132.05, 131.99, 129.08, 128.69, 128.24, 127.96, 127.47, 126.36, 124.49, 123.53, 123.09, 122.58, 120.84, 117.11, 115.09, 111.24, 94.26, 55.56 |
| 4k | 8.11(d, J=7.2 Hz, 1H), 7.83(d, J=7.6 Hz, 1H), 7.71(d, J=16.0 Hz, 1H), 7.65(t, J=7.7 Hz, 1H), 7.53(dd, J=21.2, 11.8 Hz, 2H), 7.44(dd, J=8.0, 5.0 Hz, 2H), 7.40(dd, J=14.2, 7.6 Hz, 3H), 7.21(d, J=8.0 Hz, 1H), 7.10(t, J=11.3 Hz, 1H), 7.04(t, J=8.5 Hz, 2H), 6.80(d, J=16.0 Hz, 1H), 5.32(s, 1H) | 188.30, 165.94, 164.17(d, J=252.3 Hz), 162.34, 153.81, 151.31, 142.58, 135.42, 133.21, 132.19, 130.85(d, J=2.6 Hz), 130.35(d, J=8.5 Hz), 128.85, 128.00, 127.84, 127.52, 125.28, 124.50, 123.03, 122.64, 117.18, 116.22(d, J=21.8 Hz), 115.08, 94.35 |
| 4l | 8.12(d, J=8.0 Hz, 1H), 7.83(d, J=7.8 Hz, 1H), 7.71(d, J=15.9 Hz, 1H), 7.65(t, J=7.9 Hz, 1H), 7.57(d, J=15.9 Hz, 1H), 7.51(t, J=7.7 Hz, 1H), 7.44—7.38(m, 5H), 7.21(d, J=8.1 Hz, 1H), 7.12(d, J=16.0 Hz, 1H), 6.88(d, J=8.5 Hz, 2H), 6.76(d, J=15.4 Hz, 1H), 5.33(s, 1H), 3.83(d, J=4.2 Hz, 3H) | 188.43, 165.97, 162.41, 161.86, 153.81, 151.26, 143.80, 134.85, 133.18, 131.98, 130.27, 128.87, 128.20, 128.06, 127.47, 127.29, 124.50, 123.50, 123.07, 122.60, 117.17, 115.12, 114.51, 94.31, 55.52 |
| 4m | 8.61(d, J=4.6 Hz, 1H), 8.10(d, J=7.9 Hz, 1H), 7.83(d, J=7.8 Hz, 1H), 7.77(d, J=16.0 Hz, 1H), 7.69(dd, J=8.3, 6.9 Hz, 1H), 7.62(dd, J=20.5, 11.8 Hz, 2H), 7.52(t, J=7.7 Hz, 1H), 7.44—7.36(m, 5H), 7.26(t, J=3.8 Hz, 1H), 7.20(d, J=8.1 Hz, 1H), 7.15(d, J=16.0 Hz, 1H), 5.32(s, 1H) | 188.73, 166.00, 162.39, 153.79, 153.08, 151.41, 150.25, 142.20, 136.92, 135.78, 133.17, 132.27, 128.90, 128.72, 127.98, 127.49, 125.01, 124.54, 124.52, 123.12, 122.59, 117.10, 115.10, 94.37 |
| 4n | 8.01(dd, J=7.9, 1.4 Hz, 1H), 7.78—7.70(m, 4H), 7.65—7.60(m, 3H), 7.40—7.34(m, 2H), 7.24(d, J=8.6 Hz, 2H), 7.14—7.05(m, 3H), 7.01(d, J=15.9 Hz, 1H), 5.46(s, 1H) | 188.46, 166.02, 164.21(d, J=252.1 Hz), 162.48, 153.95, 153.76, 142.52, 141.65, 133.60, 133.14, 131.00(d, J=3.0 Hz), 130.50, 130.44, 126.22, 125.13, 124.35, 123.14, 122.08, 117.05, 116.39, 116.22, 115.28, 94.00 |
| 4o | 8.01(dd, J=7.9, 1.4 Hz, 1H), 7.88(d, J=15.5 Hz, 1H), 7.74—7.70(m, 3H), 7.62(t, J=12.6 Hz, 1H), 7.42(d, J=5.1 Hz, 1H), 7.39—7.34(m, 3H), 7.23(d, J=8.6 Hz, 2H), 7.09(dd, J=5.0, 3.8 Hz, 1H), 7.03(d, J=16.0 Hz, 1H), 6.88(d, J=15.5 Hz, 1H), 5.46(s, 1H) | 188.06, 165.98, 162.41, 153.91, 153.78, 141.33, 140.28, 136.26, 133.67, 133.09, 132.17, 130.46, 129.16, 128.51, 126.45, 124.31, 124.25, 123.13, 122.01, 117.02, 115.31, 94.02 |
| 4p | 8.21(d, J=2.6 Hz, 1H), 8.19(s, 2H), 7.60(d, J=8.4 Hz, 1H), 7.35 8.02(dd, J=7.8, 1.2 Hz, 1H), 7.77(d, J=6.8 Hz, 1H), 7.75—7.72(m, 3H), 7.65—7.60(m, 3H), 7.42(dd, J=6.2, 3.8 Hz, 3H), 7.40—7.34(m, 2H), 7.24(d, J=8.6 Hz, 2H), 7.11(d, J=5.9 Hz, 1H), 7.08(d, J=5.9 Hz, 1H), 5.47(s, 1H) | 188.68, 166.00, 162.44, 153.93, 153.78, 143.83, 141.54, 134.77, 133.68, 133.11, 130.75, 130.48, 129.10, 128.55, 126.24, 125.49, 124.32, 123.14, 122.03, 117.04, 115.31, 94.03 |
| Compound | X | Ar | Inhibition rate(%) | |||
|---|---|---|---|---|---|---|
| 10 μmol/L SGC7901 | 1 μmol/L SGC7901 | 10 μmol/L HepG2 | 1 μmol/L HepG2 | |||
| 4a | 4⁃O⁃ | Pyrid⁃2⁃yl | 98.18±0.73 | 16.87±1.74 | 94.94±0.72 | 11.42±1.31 |
| 4b | 4⁃O⁃ | Pyrid⁃3⁃yl | 98.41±1.54 | 28.78±2.83 | 96.90±0.46 | 15.98±1.50 |
| 4c | 2⁃O⁃ | 2,4⁃di⁃OMePh | 98.38±0.70 | 86.98±0.72 | 93.92±0.72 | 25.42±3.31 |
| 4d | 2⁃O⁃ | Ph | 97.59±2.26 | 22.47±1.33 | 77.96±2.09 | 6.30±1.14 |
| 4e | 4⁃O⁃ | 2⁃OMePh | 95.02±4.96 | 21.23±1.06 | 96.93±0.40 | 14.55±1.83 |
| 4f | 4⁃O⁃ | 4⁃ClPh | 85.56±1.77 | 13.39±2.29 | 95.29±3.25 | 25.00±2.39 |
| 4g | 4⁃O⁃ | 3,4⁃di⁃OMePh | 0.28±0.84 | 0±3.36 | 46.06±3.96 | 19.31±1.21 |
| 4h | 4⁃O⁃ | Fural⁃2⁃yl | 88.46±1.56 | 15.49±1.80 | 51.01±5.77 | 17.43±2.63 |
| 4i | 2⁃O⁃ | Thiophen⁃2⁃yl | 98.77±3.13 | 10.98±2.35 | 86.14±3.99 | 0.00±3.01 |
| 4j | 2⁃O⁃ | 2⁃OMePh | 97.36±1.85 | 80.83±1.18 | 95.56±0.48 | 26.92±1.29 |
| 4k | 2⁃O⁃ | 4⁃FPh | 96.28±1.35 | 12.64±1.17 | 95.94±0.38 | 15.22±0.98 |
| 4l | 2⁃O⁃ | 4⁃OMePh | 96.31±1.11 | 42.73±1.71 | 91.61±0.81 | 66.69±3.54 |
| 4m | 2⁃O⁃ | Pyrid⁃2⁃yl | 97.39±6.29 | 43.69±3.28 | 99.94±0.07 | 44.52±1.62 |
| 4n | 4⁃O⁃ | 4⁃FPh | 95.26±2.74 | 5.03±1.97 | 85.80±3.20 | 7.41±1.27 |
| 4o | 4⁃O⁃ | Thiophen⁃2⁃yl | 22.12±1.49 | 15.35±3.58 | 89.65±3.29 | 8.61±0.74 |
| 4p | 4⁃O⁃ | Ph | 88.44±3.43 | 1.03±1.59 | 79.99±5.42 | 12.79±1.80 |
| Epirubicin * | 78.38±2.65 | 42.43±1.79 | 81.64±8.57 | 1.36±8.13 | ||
Table 3 Inhibition rates(%) of title compounds against SGC7901 and HepG2 cells in vitro
| Compound | X | Ar | Inhibition rate(%) | |||
|---|---|---|---|---|---|---|
| 10 μmol/L SGC7901 | 1 μmol/L SGC7901 | 10 μmol/L HepG2 | 1 μmol/L HepG2 | |||
| 4a | 4⁃O⁃ | Pyrid⁃2⁃yl | 98.18±0.73 | 16.87±1.74 | 94.94±0.72 | 11.42±1.31 |
| 4b | 4⁃O⁃ | Pyrid⁃3⁃yl | 98.41±1.54 | 28.78±2.83 | 96.90±0.46 | 15.98±1.50 |
| 4c | 2⁃O⁃ | 2,4⁃di⁃OMePh | 98.38±0.70 | 86.98±0.72 | 93.92±0.72 | 25.42±3.31 |
| 4d | 2⁃O⁃ | Ph | 97.59±2.26 | 22.47±1.33 | 77.96±2.09 | 6.30±1.14 |
| 4e | 4⁃O⁃ | 2⁃OMePh | 95.02±4.96 | 21.23±1.06 | 96.93±0.40 | 14.55±1.83 |
| 4f | 4⁃O⁃ | 4⁃ClPh | 85.56±1.77 | 13.39±2.29 | 95.29±3.25 | 25.00±2.39 |
| 4g | 4⁃O⁃ | 3,4⁃di⁃OMePh | 0.28±0.84 | 0±3.36 | 46.06±3.96 | 19.31±1.21 |
| 4h | 4⁃O⁃ | Fural⁃2⁃yl | 88.46±1.56 | 15.49±1.80 | 51.01±5.77 | 17.43±2.63 |
| 4i | 2⁃O⁃ | Thiophen⁃2⁃yl | 98.77±3.13 | 10.98±2.35 | 86.14±3.99 | 0.00±3.01 |
| 4j | 2⁃O⁃ | 2⁃OMePh | 97.36±1.85 | 80.83±1.18 | 95.56±0.48 | 26.92±1.29 |
| 4k | 2⁃O⁃ | 4⁃FPh | 96.28±1.35 | 12.64±1.17 | 95.94±0.38 | 15.22±0.98 |
| 4l | 2⁃O⁃ | 4⁃OMePh | 96.31±1.11 | 42.73±1.71 | 91.61±0.81 | 66.69±3.54 |
| 4m | 2⁃O⁃ | Pyrid⁃2⁃yl | 97.39±6.29 | 43.69±3.28 | 99.94±0.07 | 44.52±1.62 |
| 4n | 4⁃O⁃ | 4⁃FPh | 95.26±2.74 | 5.03±1.97 | 85.80±3.20 | 7.41±1.27 |
| 4o | 4⁃O⁃ | Thiophen⁃2⁃yl | 22.12±1.49 | 15.35±3.58 | 89.65±3.29 | 8.61±0.74 |
| 4p | 4⁃O⁃ | Ph | 88.44±3.43 | 1.03±1.59 | 79.99±5.42 | 12.79±1.80 |
| Epirubicin * | 78.38±2.65 | 42.43±1.79 | 81.64±8.57 | 1.36±8.13 | ||
| Compound | Cell line | Toxic regression equation | Correlation coefficient | IC50/(μmol∙L-1) |
|---|---|---|---|---|
| 4c | SGC7901 | y=1.37x+6.38 | 0.99 | 0.22±0.02 |
| 4j | y=1.18x+6.10 | 0.99 | 0.27±0.01 | |
| Epirubicin* | y=0.86x+5.12 | 0.99 | 1.23±0.07 | |
| 4l | HepG2 | y=1.03x+5.72 | 0.99 | 0.47±0.05 |
| Epirubicin* | y=1.53x+4.81 | 0.99 | 2.30±0.14 |
Table 4 IC50 values of title compounds 4c, 4j and 4l against SGC7901 or HepG2 cells
| Compound | Cell line | Toxic regression equation | Correlation coefficient | IC50/(μmol∙L-1) |
|---|---|---|---|---|
| 4c | SGC7901 | y=1.37x+6.38 | 0.99 | 0.22±0.02 |
| 4j | y=1.18x+6.10 | 0.99 | 0.27±0.01 | |
| Epirubicin* | y=0.86x+5.12 | 0.99 | 1.23±0.07 | |
| 4l | HepG2 | y=1.03x+5.72 | 0.99 | 0.47±0.05 |
| Epirubicin* | y=1.53x+4.81 | 0.99 | 2.30±0.14 |
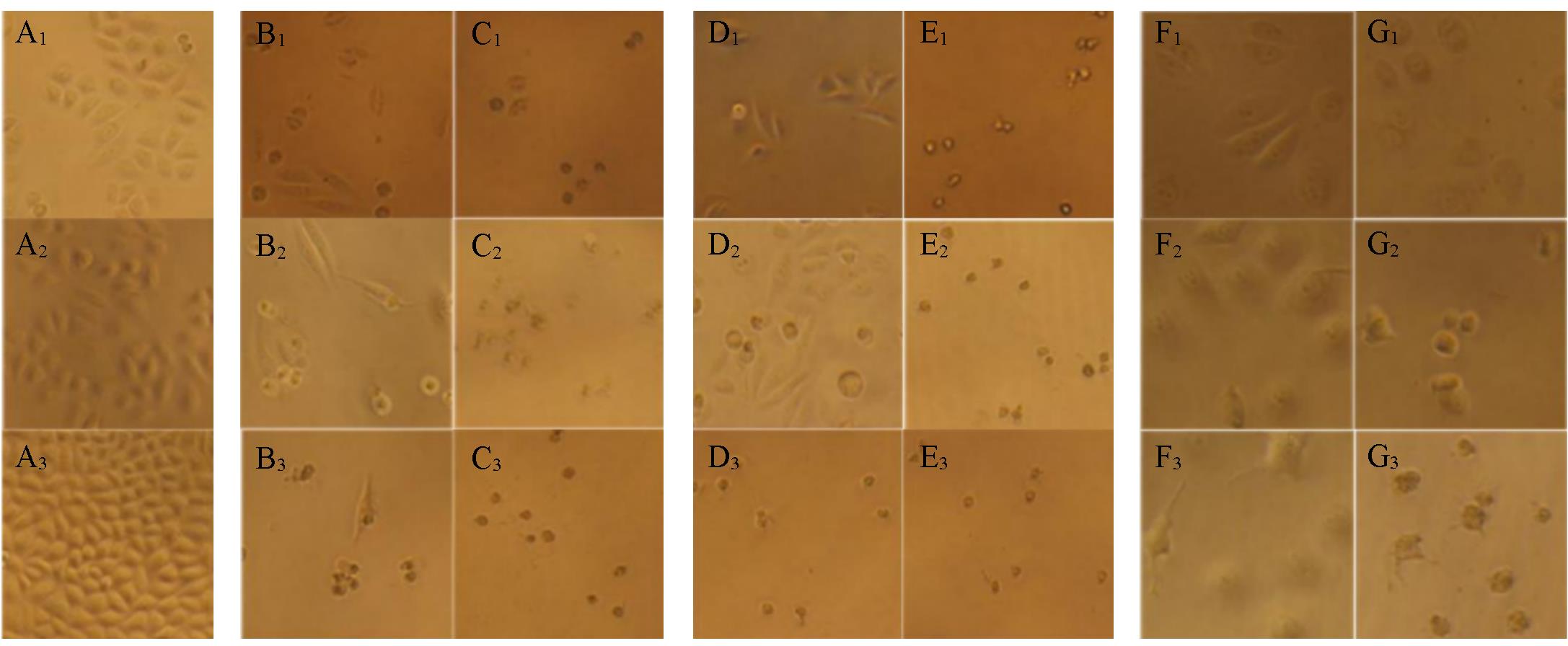
Fig.1 Morphology effects of SGC7901 cells treated with DMSO(0.1%, mass fraction)(A), compounds 4c(B, C) and 4j(D, E), and Epirubicin(F, G) with 24(A1—G1), 48(A2—G2) and 72 h(A3—G3)Concentration of 4c/4j/Epirubicin(μmol·L-1): (B, D, F) 1; (C, E, G) 10.
| 1 | Li J. W., Vederas J. C., Science, 2009, 325(5937), 161—165 |
| 2 | Newman D. J., Cragg G. M., J. Nat. Prod., 2020, 83(3), 770—803 |
| 3 | Decorte B. L., J. Med. Chem., 2016, 59(20), 9295—9304 |
| 4 | Wang X., Chai J., Gu Y., Zhang D., Meng F., Si X., Yang C., Xue W., J. Agric. Food Chem., 2022, 70(41), 13165—13175 |
| 5 | El⁃Feraly F. S., Ayalp A., Al⁃Yahya M. A., McPhail D. R., McPhail A. T., J. Nat. Prod., 1990, 53(1), 66—71 |
| 6 | Baloglu E., Kingston D. G. I., J. Nat. Prod., 1999, 62(7), 1068—1071 |
| 7 | Butler M. S., J. Nat. Prod., 2004, 67(12), 2141—2153 |
| 8 | Nelson K. M., Dahlin J. L., Bisson J., Graham J., Pauli G. F., Walters M. A., J. Med. Chem., 2017, 60(5), 1620—1637 |
| 9 | Salehi B., Stoganovic Z., Matejic J., Sharifi⁃Rad M., Anil⁃Kumar N. V., Martins N., Sharifi⁃Rad J., Eur. J. Med. Chem., 2019, 163, 527—545 |
| 10 | Anand P., Kunnumakkara A. B., Newman R. A., Aggarwal B. B., Mol. Pharmaceutics, 2007, 4(6), 807—818 |
| 11 | Zhang J. P., Li Q., Zhang C., Li P., Chen L. J., Wang Y. H., Ruan X. H., Xiao W., Xue W., Chem. Pap., 2018, 72(9), 2193—2202 |
| 12 | Wang J. Q., Wang X. B., Wang Y., Tang W. J., Shi J. B., Liu X. H., Eur. J. Med. Chem., 2018, 156, 493—509 |
| 13 | Hosseini⁃Zare M. S., Sarhadi M., Zarei M., Thilagavathi R., Selvam C., Eur. J. Med. Chem., 2021, 210(113072), 1—20 |
| 14 | Wang X., Chen M., Li Q., Zhang J., Ruan X., Xie Y., Xue W., Chem. Pap., 2017, 71(7), 1225—1233 |
| 15 | Chen L., Wang X., Tang X., Xia R., Guo T., Zhang C., Li X., Xue W., BMC Chem., 2019, 13(34), 1—12 |
| 16 | Zhang J. P., Li P., Wang Y. H., Zhang C., Chen L. J., Tang X., He M., Xue W., Chem. J. Chinese Universities, 2018, 39(7), 1455—1461 |
| 张菊平, 李普, 王一会, 张橙, 陈丽娟, 汤旭, 贺鸣, 薛伟. 高等学校化学学报, 2018, 39(7), 1455—1461 | |
| 17 | Chen L., Guo T., Xia R., Tang X., Chen Y., Zhang C., Xue W., Molecules, 2019, 24(925), 1—12 |
| 18 | Li Q., Zhang J., Chen L. Z., Wang J. Q., Zhou H. P., Tang W. J., Xue W., Liu X. H., J. Enzyme Inhib. Med. Chem., 2018, 33(1), 130—138 |
| 19 | Su S., Chen M., Li Q., Wang Y., Chen S., Sun N., Xie C., Huai Z., Huang Y., Xue W., Bioorg. Med. Chem., 2021, 32(115999), 1—12 |
| 20 | Zhou Q., Zhou Y., Zhu Y., Gong C., Wu Y., Xue W., J. Agric. Food Chem., 2022, 70(51), 16096—16105 |
| 21 | Dixon R. A., Nature, 2001, 411(6839), 843—847 |
| 22 | Zhao L., Zhang J., Liu T., Mou H., Wei C., Hu D., Song B., J. Agric. Food Chem., 2020, 68(4), 975—981 |
| 23 | Saudhu S., Bansal Y., Silakari O., Bansal G., Bioorg. Med. Chem., 2014, 22, 3806—3814 |
| 24 | Ren Y., Song X., Tan L., Guo C., Wang M., Liu H., Cao Z., Li Z., Peng C., Front. Pharmacol., 2020, 11(571535), 1—18 |
| 25 | Wu A., Lu J., Zhong G., Lu L., Qu Y., Zhang C., Phytother. Res., 2022, 36(10), 3805—3832 |
| 26 | Liang Y., Xie L., Liu K., Cao Y., Dai X., Wang X., Lu J., Zhang X., Li X., Phytother. Res., 2021, 35(11), 6131—6147 |
| 27 | Nasser M. I., Zhu S., Hu H., Huang H., Guo M., Zhu P.,Biomed. Pharmacother., 2019, 120(109401), 1—8 |
| 28 | Yang S., Dai W., Wang J., Zhang X., Zheng Y., Bi S., Pang L., Ren T., Yang Y., Sun Y., Zheng Z., Wu S., Kong J., Front. Pharmacol., 2022, 13(945627), 1—10 |
| 29 | Wu Y., Xu J., Liu Y., Zeng Y., Wu G., Front. Oncol., 2022, 10(592853), 1—11 |
| 30 | Al⁃Warhi T., Sabt A., Elkaeed E. B., Eldehna W. M., Bioorg. Med., 2020, 103(104163), 1—15 |
| 31 | Ruan X. H., Zhao H. J., Zhang C., Chen L. J., Li P., Wang Y. H., He M., Xue W., Chem. J. Chinese Universities, 2018, 39(6), 1197—1204 |
| 阮祥辉, 赵洪菊, 张橙, 陈丽娟, 李普, 王一会, 贺鸣, 薛伟. 高等学校化学学报, 2018, 39(6), 1197—1204 | |
| 32 | Xiao Y. H., Zhang G. J., Zong L., Liu G. H., Ren L. J., Dong J. X., Chem. J. Chinese Universities, 2019, 40(9), 1897—1903 |
| 肖艳华, 张广杰, 宗良, 刘国宏, 任丽君, 董俊兴. 高等学校化学学报, 2019, 40(9), 1897—1903 |
| [1] | 苏丽娇, 保秋连, 杨云汉, 罗建萍, 杨举, 张郡童, 陶欣, 钏永明, 杨丽娟. 香豆素功能化柱[5]芳烃对Fe3+和Cu2+的荧光传感[J]. 高等学校化学学报, 2023, 44(4): 20220549. |
| [2] | 唐倩, 但飞君, 郭涛, 兰海闯. 喹啉酮-香豆素类Hg2+比色荧光探针的合成及应用[J]. 高等学校化学学报, 2022, 43(2): 20210660. |
| [3] | 李伦, 张静妍, 罗静, 刘仁, 朱乙. UV/Vis-LED激发的香豆素吡啶鎓盐光引发剂的合成及性能[J]. 高等学校化学学报, 2022, 43(10): 20220178. |
| [4] | 赵慧君, 吴通, 孙玥, 段炼, 马嫣宇. 基于香豆素的比率型荧光探针对溶液及气相中三氟化硼的检测[J]. 高等学校化学学报, 2021, 42(8): 2422. |
| [5] | 匡小军, 伊京伟, 方晓霞, 赖东梅, 徐宏. 水溶性香豆素荧光底物的制备及在液滴数字式检测中的应用[J]. 高等学校化学学报, 2021, 42(11): 3537. |
| [6] | 肖艳华, 张广杰, 宗良, 刘国宏, 任丽君, 董俊兴. 开口箭化学成分及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(9): 1897. |
| [7] | 马玉坤, 王海君, 郭梦岩. 狼毒乙素分子印迹膜荧光传感器的制备及在中药材检测中的应用[J]. 高等学校化学学报, 2019, 40(7): 1381. |
| [8] | 韩涛, 蔡小霞, 李聪, 乔从德, 赵辉. 生物基氢化香豆素增韧环氧树脂的制备及性能[J]. 高等学校化学学报, 2019, 40(5): 1043. |
| [9] | 吕明君, 李雯, 杨新颖, 方浩. N9位芳基取代嘌呤-8-酮类衍生物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(2): 254. |
| [10] | 方芳,薛良敏,丛婧,田超,王孝伟,刘俊义,张志丽. 2-位或4-位取代吡啶并嘧啶类非经典叶酸拮抗剂的合成及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(10): 2111. |
| [11] | 张培全,杨倩倩,龙惠丹,陈鑫. 金诺芬衍生物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(10): 2097. |
| [12] | 刘莉, 马洋洋, 王宽, 贾云静, 李婉, 朱华结. β-咔啉衍生物的抗肿瘤及抗菌活性[J]. 高等学校化学学报, 2018, 39(4): 674. |
| [13] | 韩瑞霞, 吕继涛, 张淑贞. 一种适用于复杂异相体系中羟基自由基定量检测的探针分子—香豆素[J]. 高等学校化学学报, 2018, 39(12): 2658. |
| [14] | 王磊, 郑国钧, 季奇, 陈博, 巩龙龙, 高聪敏, 杜镇建, 张兴民. PI3K/mTOR抑制剂的合成及生物活性[J]. 高等学校化学学报, 2017, 38(9): 1590. |
| [15] | 白信法, 马宣, 解晓霞, 邵明莎, 郭宁宁, 严宁, 姚雷. 微管菌素类似物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2017, 38(1): 47. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||