

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (11): 2426.doi: 10.7503/cjcu20209236
• 庆祝《高等学校化学学报》复刊40周年专栏 • 上一篇 下一篇
吴倩,程丹,吕芸,袁林,张晓兵
收稿日期:2020-07-18
出版日期:2020-11-10
发布日期:2020-11-06
基金资助:
WU Qian, CHENG Dan, LÜ Yun, YUAN Lin( ), ZHANG Xiaobing
), ZHANG Xiaobing
Received:2020-07-18
Online:2020-11-10
Published:2020-11-06
Contact:
YUAN Lin
E-mail:lyuan@hnu.edu.cn
Supported by:摘要:
肝损伤是影响公众健康的重大问题之一, 已经引起了人们越来越多的关注. 而过表达的过氧化亚硝酸盐(ONOO?)在肝损伤等疾病的发病机制中起着重要作用, 被认为是一种与早期肝损伤密切相关的生物活性分子. 因此, 为了探究ONOO?在肝损伤过程中的作用, 开发可以实现肝损伤过程中ONOO?高选择性和实时检测的分析方法具有重要意义. 本文报道了一种具有大斯托克斯位移的远红光至近红外(FR-NIR)ONOO?荧光探针. 由于该探针具有大的斯托克斯位移, 可以有效消除光谱重叠和自吸收的干扰, 从而显著提高成像的信噪比. 此外, 该探针对ONOO?具有高的灵敏度(检出限为25.8 nmol/L)和良好的选择性, 被成功用于药物诱导肝损伤过程中ONOO?信号的成像检测.
中图分类号:
TrendMD:
吴倩, 程丹, 吕芸, 袁林, 张晓兵. 大斯托克斯位移远红光至近红外荧光探针用于检测肝损伤过程中过氧化亚硝酸盐的动态变化. 高等学校化学学报, 2020, 41(11): 2426.
WU Qian, CHENG Dan, LÜ Yun, YUAN Lin, ZHANG Xiaobing. Monitoring of Peroxynitrite Variation During Liver Injury Adopting a Far Red to Near-infrared Fluorescent Probe with Large Stokes Shift. Chem. J. Chinese Universities, 2020, 41(11): 2426.
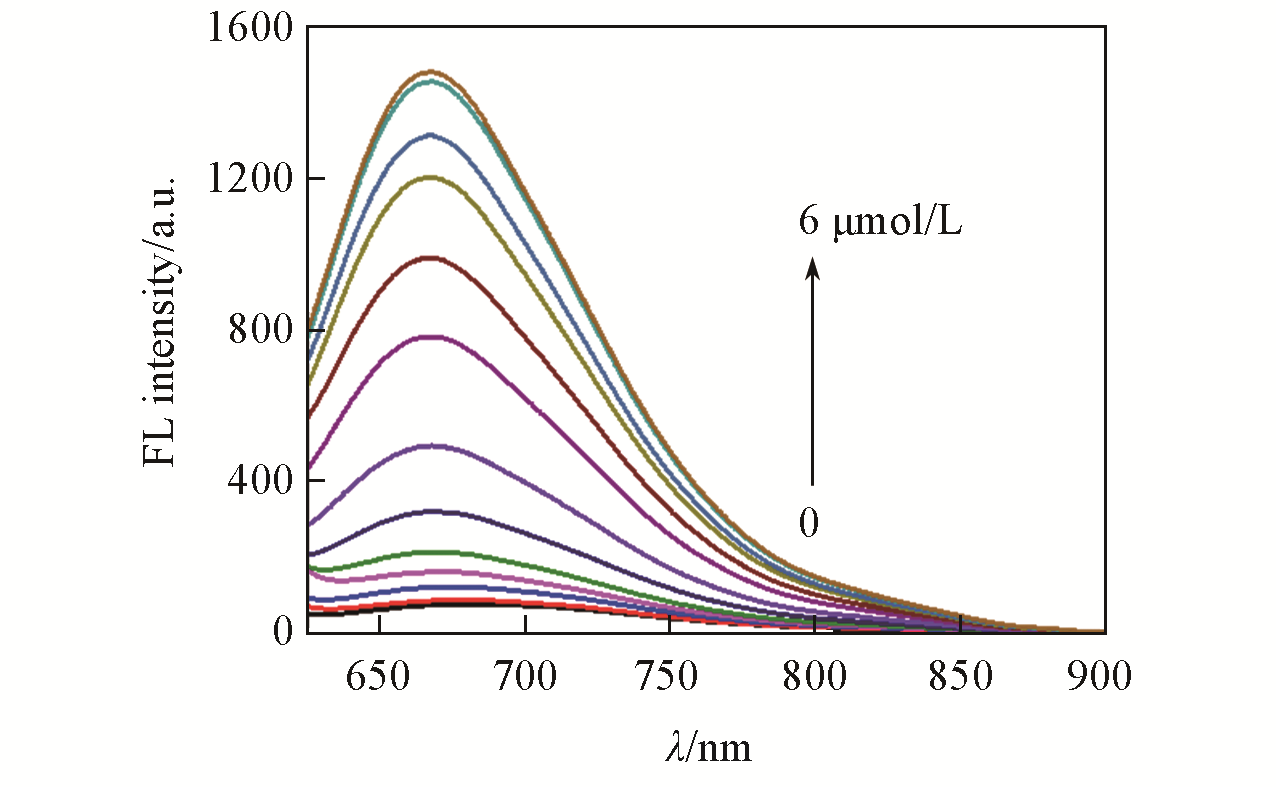
Fig.1 Fluorescence spectra of LSDQ?ONOO-(5 μmol/L) upon addition of ONOO- (0—6 μmol/L) in PBS/EtOH(volume ratio 8∶2, pH=7.4) buffer solutionnote:The mixture was kept for 20 min at 37 ℃ before the fluorescence intensity of the LSDQ?ONOO- solution was recorded. λex=580 nm.
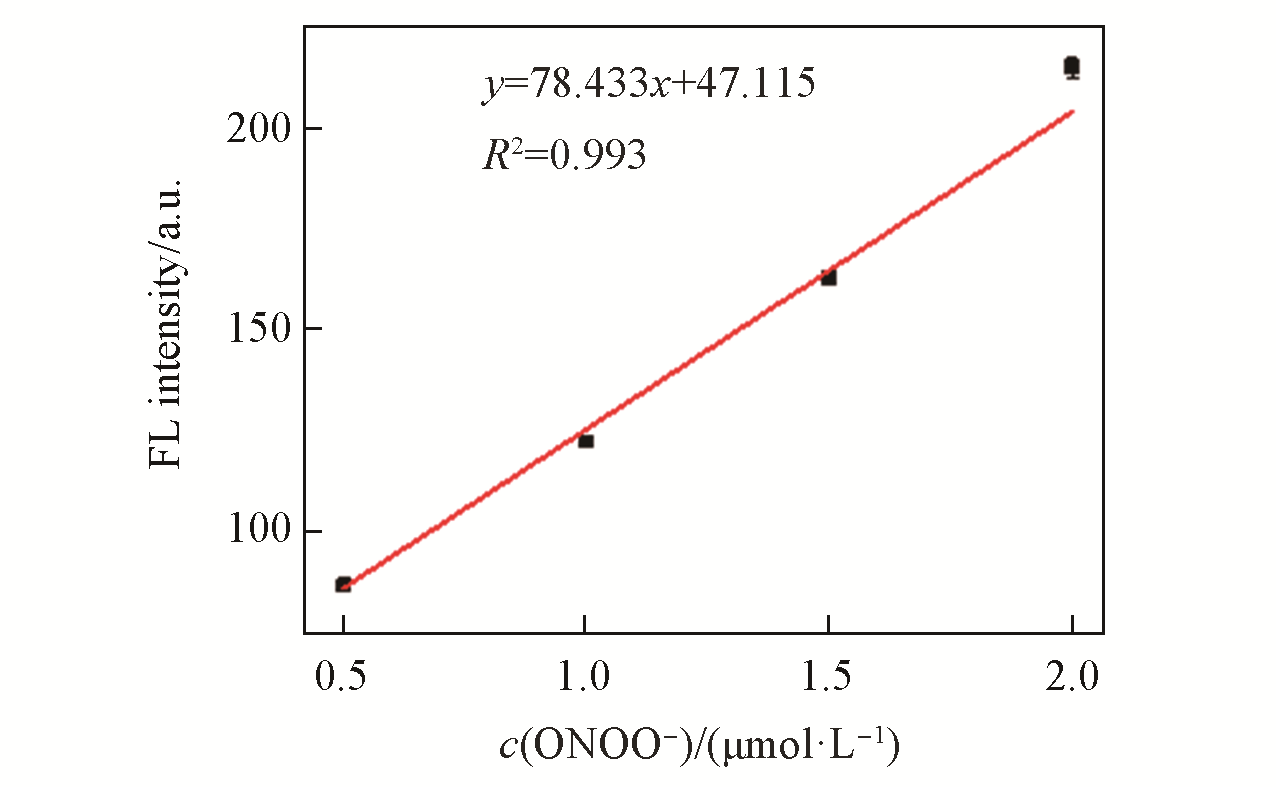
Fig.2 Linear correlation between the FL intensity of LSDQ?ONOO-(5 μmol/L) and ONOO- concentration in PBS/EtOH(volume ratio 8∶2, pH=7.4) buffer solutionnote:The mixture was kept for 20 min at 37 ℃ before the fluorescence intensity of the LSDQ?ONOO- solution was recorded. λex=580 nm.
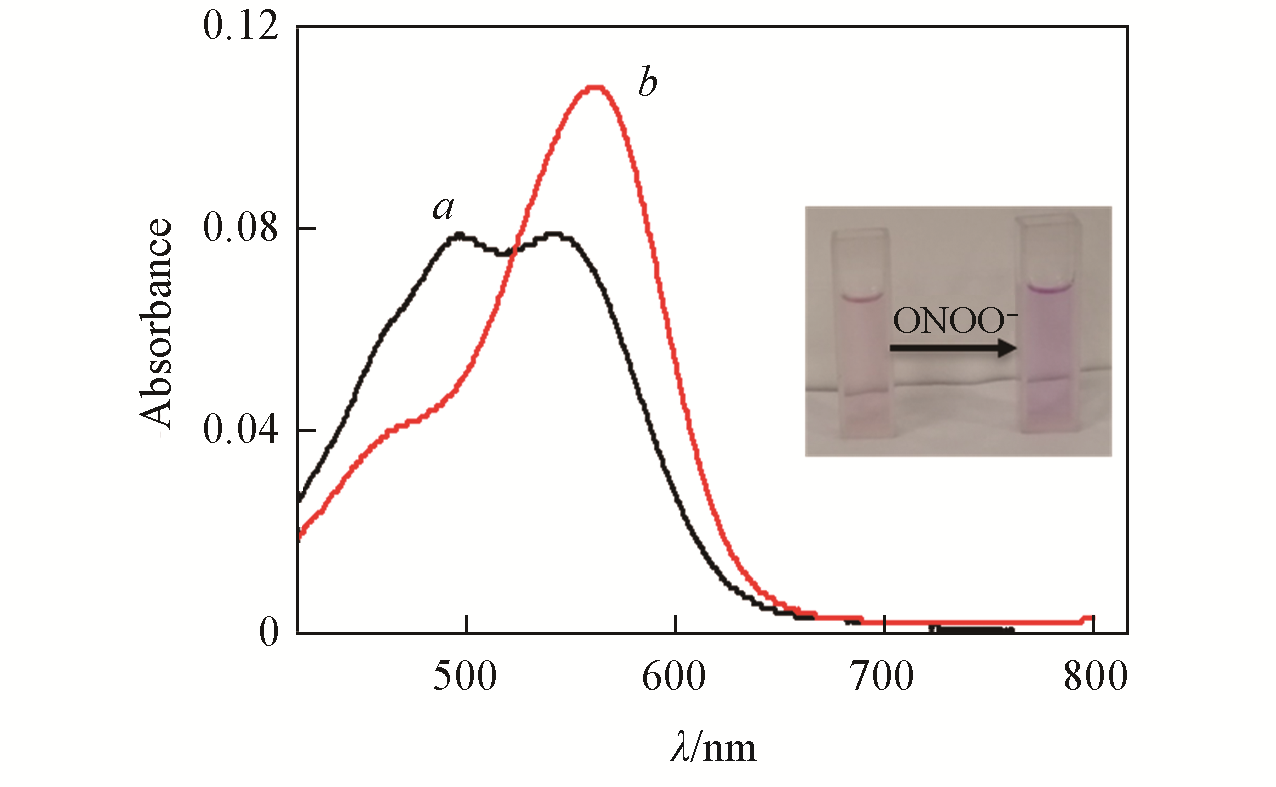
Fig.3 Absorption spectra of LSDQ?ONOO-(5 μmol/L) in the absence(a) and in the presence(b) of ONOO-(5 μmol/L) in PBS/EtOH(volume ratio 8∶2, pH=7.4) buffer solutionnote:The mixture was kept for 20 min at 37 ℃ before the absorption of the LSDQ?ONOO- solution was recorded. Inset: after reacting with ONOO?, the absorption peak of the probe shifted to 562 nm; due to the fluorescence recovery of the probe, the solution color changed from light red to dark red.
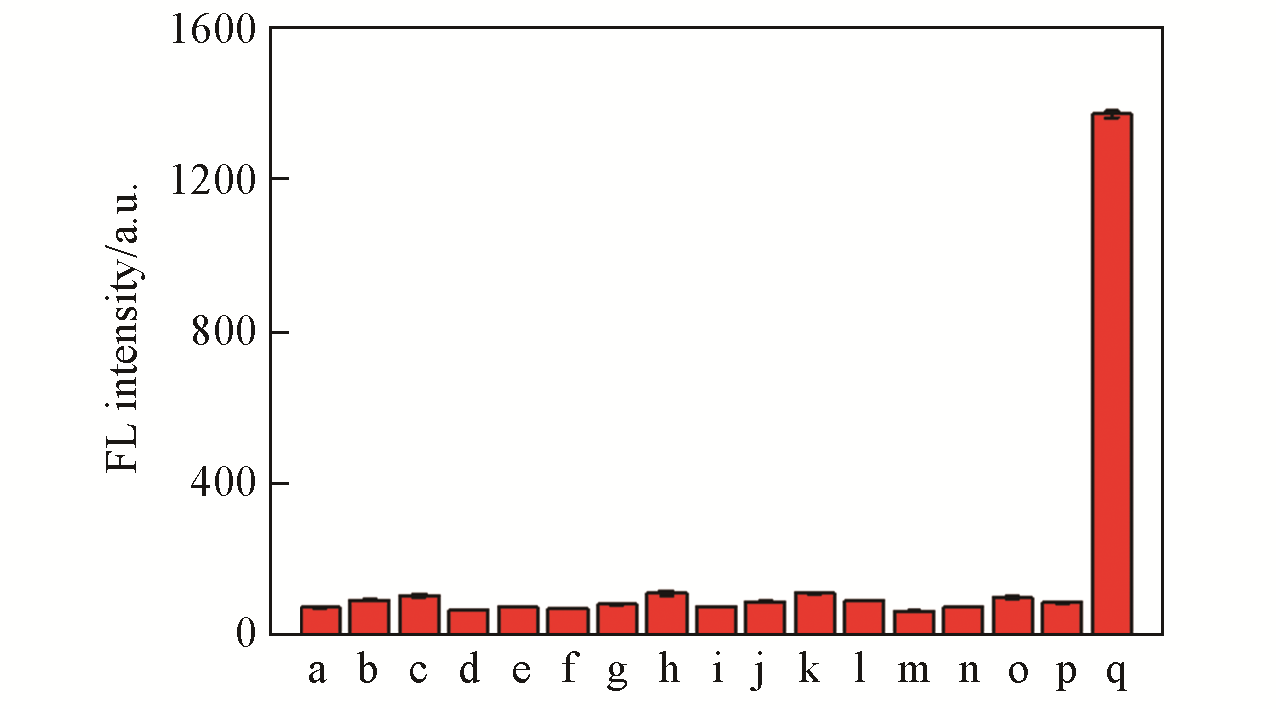
Fig.4 Fluorescence intensity of LSDQ?ONOO-(5 μmol/L) in PBS/EtOH(volume ratio 8∶2, pH=7.4) buffer solu? tion toward various analytes(100 μmol/L)note:1 mmol/L for GSH, 50 μmol/L for HOCl, 5 μmol/L for ONOO?. a. Blank; b. Cu2+; c. Fe2+; d. K+; e. Na+; f. Mg2+; g. SO42-; h. GSH; i. Cys; j. H2S; k. ·OH; l. NO2-; m. O2·-; n. H2O2; o. HOCl; p. t?BuOOH; q. ONOO-. The mixture was kept for 20 min at 37 ℃ before the fluorescence intensity of the LSDQ?ONOO- solution was recorded. λex=580 nm.
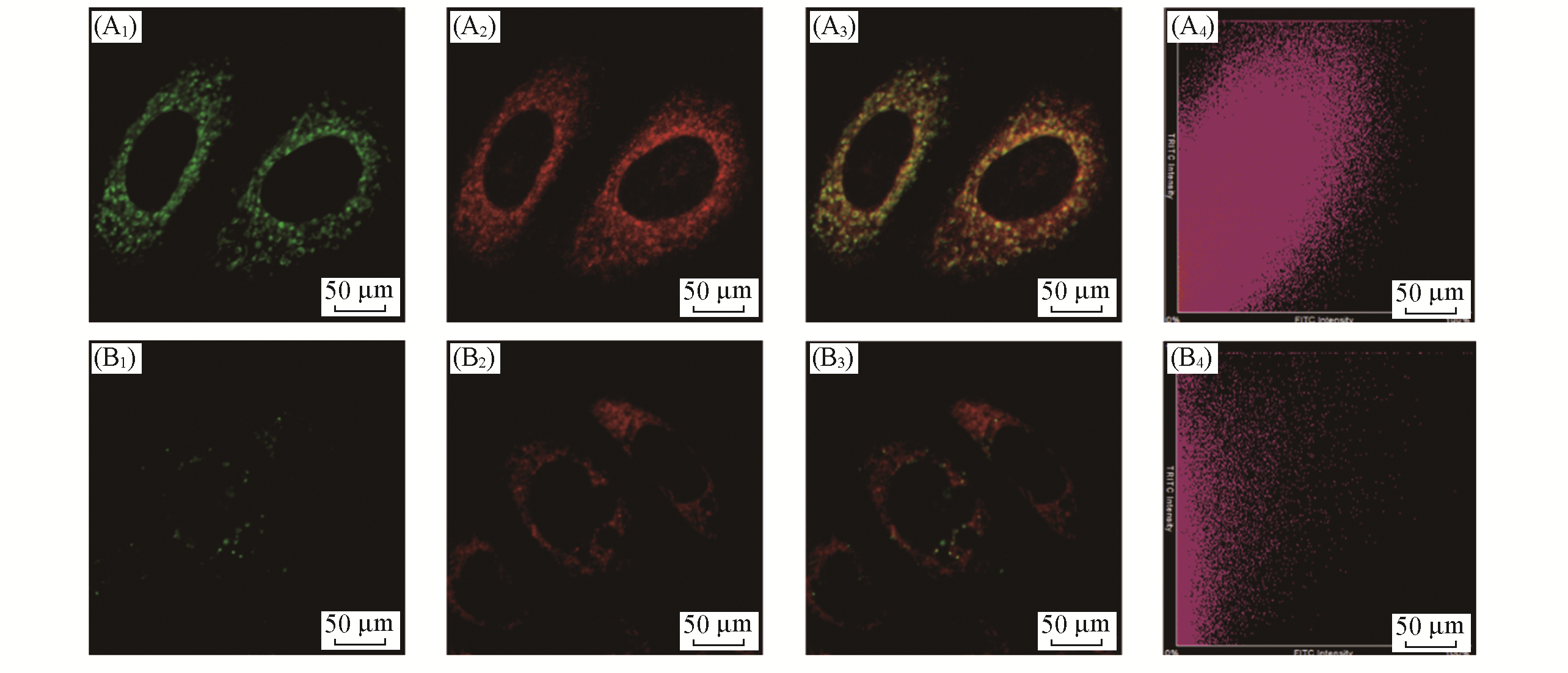
Fig.5 Distribution of LSDQ?ONOO- in HepG2 cellsnote:HepG2 cells were pretreated with LPS(1 μg/mL) and IFN?γ(50 ng/mL) for 12 h, and then incubated with 5 μmol/L LSDQ?ONOO- for 30 min and subsequently incubated with 1 μmol/L Mito?Tracker Green(or 1 μmol/L Lyso?Tracker Green) for 10 min. Green channel: Mito?Tracker Green(λex=488 nm, λem=500―550 nm) and Lyso?Tracker Green fluorescence(λex=488 nm, λem=500―550 nm); red channel: probe fluorescence(λex=561 nm, λem=640―750 nm); yellow: merged signal. (A1) Mito?tracker green; (B1) lyso?tracker green; (A2, B2) probe; (A3, B3) overlap; (A4, B4) scatter plot.
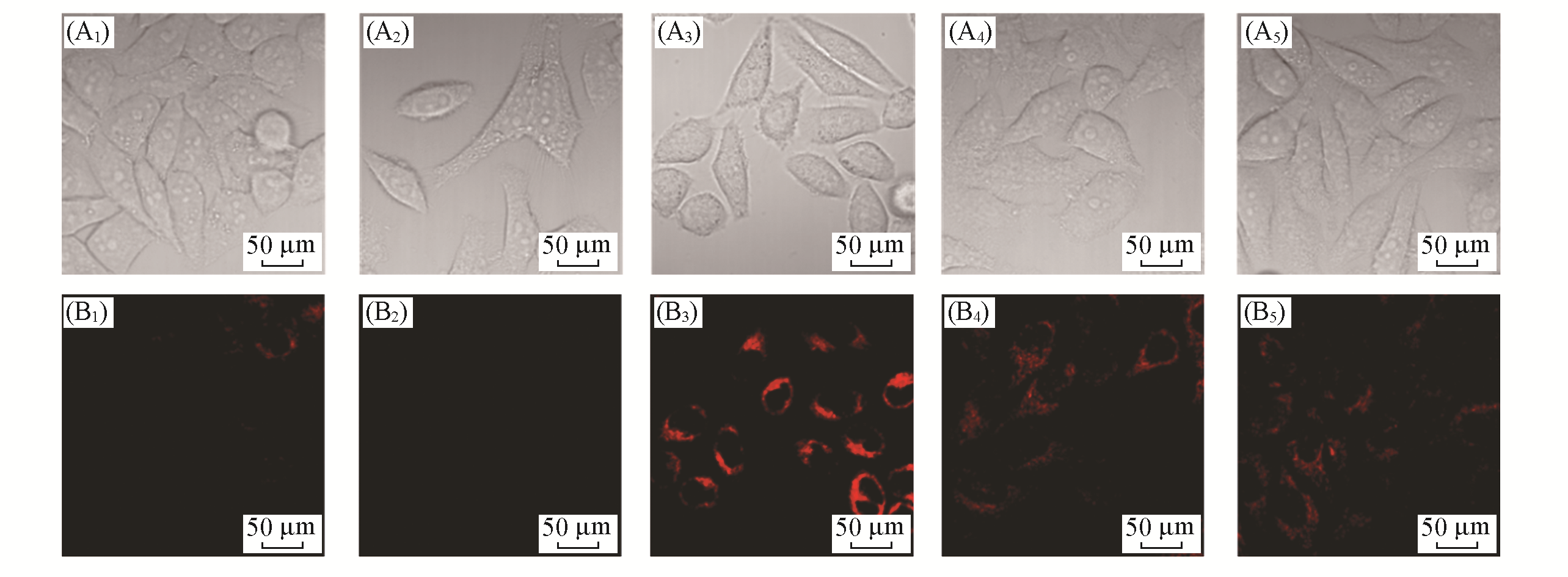
Fig.6 Fluorescent images of LSDQ?ONOO- in HepG2 cells under different conditionsnote:λex=561 nm, λem=640―750 nm. (A1―A5) Bright field; (B1―B5) red channel.(A1, B1) Cells were incubated with probe LSDQ?ONOO- (5 μmol/L, 30 min) and then imaged; (A2, B2) cells were pretreated with probe LSDQ?ONOO-(5 μmol/L, 30 min), subsequently incuba?ted with H2O2(100 μmol/L) for 30 min and then imaged; (A3, B3) cells were pre?stimulated with LPS(1 μg/mL) and IFN?γ (50 ng/mL) for 12 h, subsequently incubated with probe LSDQ?ONOO-(5 μmol/L, 30 min), and then imaged; (A4, A5, B4, B5) cells were pretreated with nitric oxide synthase inhibitor AG(5 mmol/L)(A4, B4) or O2·- scavenger TEMPO(300 μmol/L)(A5, B5) during stimulation with LPS (1 μg/mL) and IFN?γ(50 ng/mL) for 12 h, subsequently incubated with probe LSDQ?ONOO-(5 μmol/L, 30 min), and then imaged.
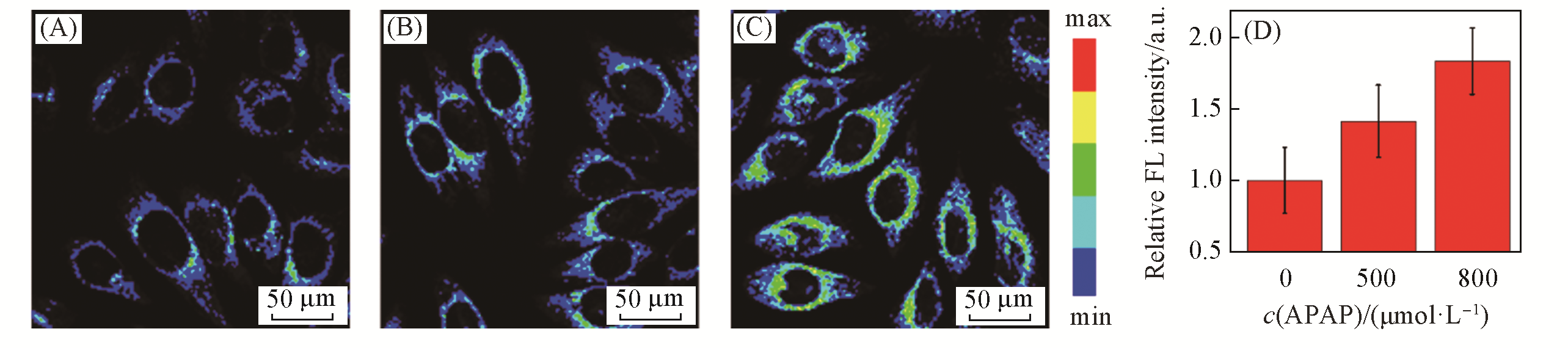
Fig.7 Fluorescent images of LSDQ?ONOO- in HepG2 cellsnote:(A—C) HepG2 cells were pretreated with different concentrations of APAP for 12 h and then incubated with 5 μmol/L LSDQ?ONOO- for 30 min. c(APAP)/(μmol·L?1): (A) 0; (B) 500; (C) 800. (D) Average intensity in panels(A—C). Data are expressed as mean ± standard deviation(SD) of three experiments. λex=561 nm, λem=640—750 nm.
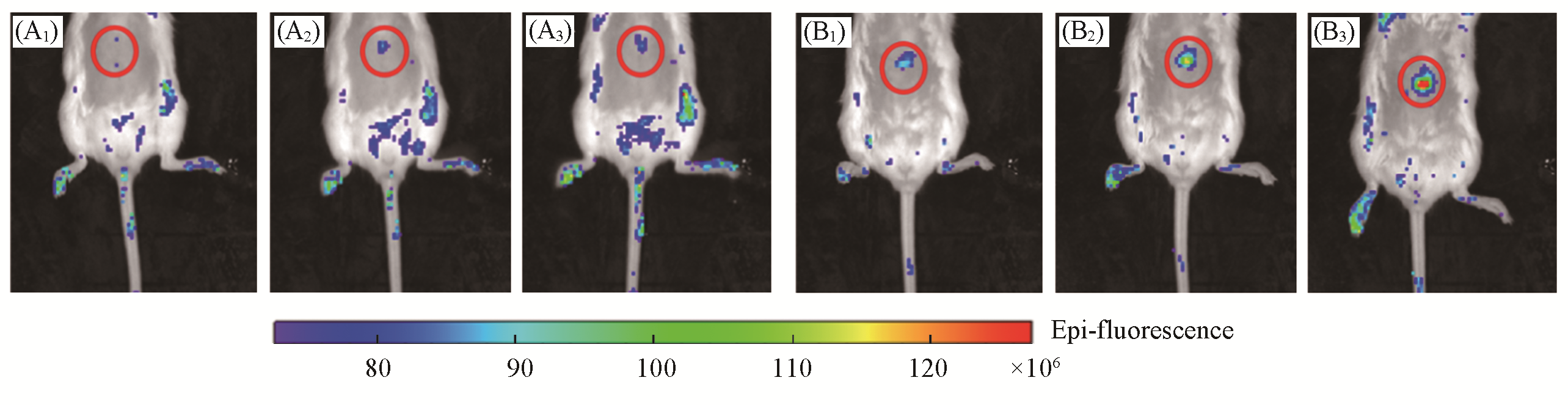
Fig.8 Representative images of BALB/c mice receiving saline(control, A1—A3), or APAP(300 mg/kg, intraperitoneally, B1—B3) followed by LSDQ?ONOO-(50 μL, 100 μmol/L, intravenously) in 30(A1, B1), 60(A2, B2) and 90 min(A3, B3)
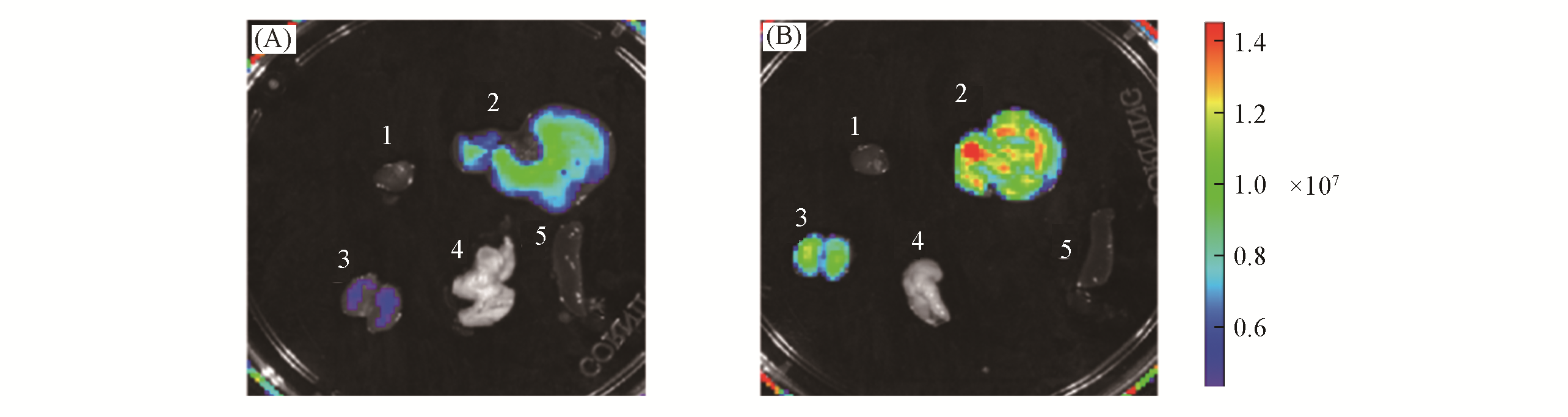
Fig.9 Fluorescence images of representative organs of BALB/c mice receiving saline(control, A) or APAP(300 mg/kg, intraperitoneally, B) followed by LSDQ?ONOO?(50 μL, 100 μmol/L, intravenously) after 90 minnote:Organs: 1. heart; 2. liver; 3. kidney; 4. lung; 5. spleen. λex=570 nm, λem=695―770 nm.
| 1 | Arnold D. T., Bentham L. M., Jacob R. P., Lilford R. J., Girling A. J., Bmc Fam. Pract., 2011, 12, 9 |
| 2 | Han X. Y., Wang R., Song X. Y., Yu F. B., Chen L. X., Anal. Chem., 2018, 90, 8108—8115 |
| 3 | Guo L. F., Tian M. G., Feng R. Q., Zhang G., Zhang R. Y., Li X. C., Liu Z. Q., He X. Q., Sun J. Z., Yu X. Q., ACS Appl. Mater.Interfaces, 2018, 10, 10706—10717 |
| 4 | Li W., Wang L., Yin S. L., Lai H. H., Yuan L., Zhang X. B., Chem. Sci., 2020, 11, 7991—7999 |
| 5 | Gao B., Li Z. T., Xue D.B ., Zhang W. H., Med. Hypotheses,2014, 82, 282—285 |
| 6 | Chen M., Vijay V., Shi Q., Liu Z., Fang H., Tong W., Drug Discov. Today, 2011, 16, 697—703 |
| 7 | Yuan L., Kaplowitz N., Clin. Liver Dis., 2013,17, 507—518 |
| 8 | Cheng D., Peng J., Lv Y., Su D., Liu D., Chen M., Yuan L., Zhang X., J. Am. Chem. Soc., 2019, 141, 6352—6361 |
| 9 | Cover C., Mansouri A., Knight T.R., Bajt M.L., Lemasters J.J., Pessayre D., Jaeschke H., J. Pharmacol. Exp. Ther., 2005, 315, 879—887 |
| 10 | Cheng D., Gong X. Y., Wu Q., Yuan J., Lv Y., Yuan L., Zhang X. B., Anal. Chem., 2020, 92, 11396—11404 |
| 11 | Li D., Wang S., Lei Z., Sun C., El⁃Toni A. M., Alhoshan M. S., Fan Y., Zhang F., Anal. Chem., 2019, 91, 4771—4779 |
| 12 | Li Y., Xie X., Yang X., Li M., Jiao X., Sun Y., Wang X., Tang B., Chem. Sci., 2017, 8, 4006—4011 |
| 13 | Zeng X. D., Chen Z. Y., Tang L., Yang H., Liu N., Zhou H., Li Y., Wu J. Z., Deng Z. X., Yu Y., Deng H., Hong X. C., Xiao Y. L., Chem. Commun., 2019, 55, 2541—2544 |
| 14 | Pacher P., Beckman J. S., Liaudet L., Physiol. Rev., 2007, 87, 315—424 |
| 15 | Ducrocq C., Blanchard B., Pignatelli B., Ohshima H., Cell. Mol. Life Sci., 1999, 55, 1068—1077 |
| 16 | Radi R., J. Biol. Chem., 2013, 288, 26464—26472 |
| 17 | Bezner B. J., Ryan L. S., Lippert A. R., Anal. Chem., 2020, 92, 309—326 |
| 18 | Masumoto H., Kissner R., Koppenol W. H., Sies H., FEBS Lett., 1996, 398, 179—182 |
| 19 | Chen X., Wang F., Hyun J. Y., Wei T., Qiang J., Ren X., Shin I., Yoon J., Chem. Soc. Rev., 2016, 45, 2976—3016 |
| 20 | Li X., Gao X., Shi W., Ma H., Chem. Rev., 2014, 114, 590—659 |
| 21 | Wang J., Guo J., Dou L., Wang R., Song Y., Yang Q., Du J., Li Y., Chem. Res. Chinese Universities, 2019, 35(4), 570—576 |
| 22 | Mi Z., Chen Y., Chen X., Yan L., Gu Q., Zhang H., Chen C., Zhang Y., Chem. Res. Chinese Universities, 2018, 34(3), 369—374 |
| 23 | Breen C., Pal R., Elsegood M. R. J., Teat S. J., Iza F., Wende K., Buckley B. R., Butler S. J., Chem. Sci., 2020, 11, 3164—3170 |
| 24 | Guo Y., Lu G., Zhuo J., Wang J., Li X., Zhang Z., J. Mater. Chem. B., 2018, 6, 2489—2496 |
| 25 | Hou J. T., Wang B., Zhang Y., Cui B., Cao X., Zhang M., Ye Y., Wang S., Chem. Commun., 2020, 56, 2759—2762 |
| 26 | Hu J. S., Shao C., Wang X., Di X., Xue X., Su Z., Zhao J., Zhu H. L., Liu H. K., Qian Y., Adv. Sci., 2019, 6, 1900341 |
| 27 | Li Y., Zhao Z., Xiao Y., Wang X., Jiao X., Xie X., Zhang J., Tang B., Anal. Chem., 2019, 91, 6097—6102 |
| 28 | Sedgwick A. C., Dou W. T., Jiao J. B., Wu L., Williams G. T., Jenkins A. T. A., Bull S. D., Sessler J. L., He X. P., James T. D., J. Am. Chem. Soc., 2018, 140, 14267—14271 |
| 29 | Sedgwick A. C., Han H. H., Gardiner J. E., Bull S. D., He X. P., James T. D., Chem. Sci., 2018, 9, 3672—3676 |
| 30 | Zhang J., Liang M., Wang X., Li Y., Jiao X., Xie X., Tang B., Chem. Commun., 2019, 55, 6719—6722 |
| 31 | Zhou D. Y., Li Y., Jiang W. L., Tian Y., Fei J., Li C. Y., Chem. Commun., 2018, 54, 11590—11593 |
| 32 | Xie X., Liu G., Su X., Li Y., Liu Y., Jiao X., Wang X., Tang B., Anal. Chem., 2019, 91, 6872—6879 |
| 33 | Cheng D., Xu W., Yuan L., Zhang X., Anal. Chem., 2017,89, 7693—7700 |
| 34 | Sedgwick A. C., Han H. H., Gardiner J. E., Bull S. D., He X. P., James T. D., Chem. Commun., 2017, 53, 12822—12825 |
| 35 | Pak Y. L., Park S. J., Wu D., Cheon B., Kim H. M., Bouffard J., Yoon J., Angew. Chem. Int. Ed., 2018, 57, 1567—1571 |
| 36 | Cheng P., Miao Q., Li J., Huang J., Xie C., Pu K., J. Am. Chem. Soc., 2019, 141, 10581—10584 |
| 37 | Kiang T. K. L., Teng X. W., Surendradoss J., Karagiozov S., Abbott F. S., Chang T. K. H., Toxicol. Appl. Pharmacol., 2011, 252, 318—324 |
| 38 | Ai X., Wang Z., Cheong H., Wang Y., Zhang R., Lin J., Zheng Y., Gao M., Xing B., Nat. Commun., 2019, 10, 1—11 |
| 39 | Ren T. B., Xu W., Zhang W., Zhang X. X., Wang Z. Y., Xiang Z., Yuan L., Zhang X. B., J. Am. Chem. Soc., 2018, 140, 7716—7722 |
| 40 | Xie X., Tang F., Liu G., Li Y., Su X., Jiao X., Wang X., Tang B., Anal. Chem., 2018, 90, 11629—11635 |
| 41 | Zhou R. H., Lu X. M., Yang Q., Wu P., Chin. Chem. Lett., 2019, 30, 1843—1848 |
| 42 | Lejeune D., Hasanuzzaman M., Pitcock A., Francis J., Sehgal I., Mol. Cancer, 2006, 5, 1—5 |
| 43 | Campbell I. L., J. Neuroimmunol., 1996, 71, 31—36 |
| [1] | 赵永梅, 穆叶舒, 洪琛, 罗稳, 田智勇. 双萘酰亚胺衍生物用于检测水溶液中的苦味酸[J]. 高等学校化学学报, 2022, 43(3): 20210765. |
| [2] | 唐倩, 但飞君, 郭涛, 兰海闯. 喹啉酮-香豆素类Hg2+比色荧光探针的合成及应用[J]. 高等学校化学学报, 2022, 43(2): 20210660. |
| [3] | 王迪, 钟克利, 汤立军, 侯淑华, 吕春欣. 席夫碱共价有机框架的合成及对I ‒ 的识别[J]. 高等学校化学学报, 2022, 43(10): 20220115. |
| [4] | 黄珊, 姚建东, 宁淦, 肖琦, 刘义. 石墨烯量子点荧光探针对碱性磷酸酶活性的高效检测[J]. 高等学校化学学报, 2021, 42(8): 2412. |
| [5] | 李安然, 赵冰, 阚伟, 宋天舒, 孔祥东, 卜凡强, 孙立, 殷广明, 王丽艳. 基于菲并咪唑的ON⁃OFF⁃ON双比色荧光探针及细胞成像[J]. 高等学校化学学报, 2021, 42(8): 2403. |
| [6] | 杨新杰, 赖艳琼, 李秋旸, 张艳丽, 王红斌, 庞鹏飞, 杨文荣. 基于环状DNA-银纳米簇荧光探针对微囊藻毒素-LR的传感检测[J]. 高等学校化学学报, 2021, 42(12): 3600. |
| [7] | 谌委菊, 陈诗雅, 薛曹叶, 刘波, 郑晶. 缺氧响应荧光探针的成像及治疗应用[J]. 高等学校化学学报, 2021, 42(11): 3433. |
| [8] | 黄加玲,刘凤娇,王婷婷,刘翠娥,郑凤英,王振红,李顺兴. 氮硫共掺杂碳量子点对胃液pH值的精确检测[J]. 高等学校化学学报, 2020, 41(7): 1513. |
| [9] | 王金金, 戚少龙, 杜建时, 杨清彪, 宋岩, 李耀先. 苯并噻唑类荧光探针的合成及对N2H4·H2O和HS |
| [10] | 张勇,申城,幸志荣,陈归柒,卢资,侯志兵,陈雪梅. 可视化检测次氯酸的苯并咪唑类荧光增强型探针[J]. 高等学校化学学报, 2019, 40(12): 2480. |
| [11] | 张莹莹,黄译文,赵冰,王丽艳,宋波. Cr 3+比色荧光探针的合成及细胞成像应用[J]. 高等学校化学学报, 2019, 40(12): 2486. |
| [12] | 喻照川, 马文辉, 吴涛, 问婧, 张永, 王丽艳, 初红涛. B,N,S共掺杂石墨烯量子点的制备及对Fe 3+和H2P |
| [13] | 刘大海, 张雪妍, 冯玉沙, 杜显龙, 薛龙启, 杜建时, 张桂荣, 杨清彪, 李耀先. 基于荧光素硫代酰肼类Hg2+荧光探针的合成及在生物成像中的应用[J]. 高等学校化学学报, 2018, 39(7): 1412. |
| [14] | 黄池宝, 潘淇, 陈华仕, 梁兴, 吕国岭. 双氰基二苯代乙烯型双光子荧光铅离子探针[J]. 高等学校化学学报, 2018, 39(5): 897. |
| [15] | 杨梅, 刘青, 唐青, 王成会, 杨梅香, 孙涛, 黄英, 陶朱. 水溶性超分子荧光探针对多菌灵的识别及细胞成像[J]. 高等学校化学学报, 2018, 39(12): 2665. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||