

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (12): 2725.doi: 10.7503/cjcu20200364
咸国轩, 于玉娥, 陈玉倩, 万小雨, 王素娜( ), 卢静(
), 卢静( )
)
收稿日期:2020-06-18
出版日期:2020-12-10
发布日期:2020-12-09
通讯作者:
王素娜,卢静
E-mail:wangsuna@lcu.edu.cn;lujing@lcu.edu.cn
基金资助:
XIAN Guoxuan, YU Yu’e, CHEN Yuqian, WAN Xiaoyu, WANG Suna( ), LU Jing(
), LU Jing( )
)
Received:2020-06-18
Online:2020-12-10
Published:2020-12-09
Contact:
WANG Suna,LU Jing
E-mail:wangsuna@lcu.edu.cn;lujing@lcu.edu.cn
Supported by:摘要:
将柔性苄氨基三羧酸配体5-(3-羧基-4-甲氧基苄氨基)间苯二甲酸(H3L)与硝酸镉和不同含氮配体在溶剂热条件下反应, 制得了配合物{[Cd(HL)(bpea)·H2O]·H2O·DMF}n(1)、 {[Cd(HL)(bpp)·H2O]·2H2O·DMF}n(2)和 {[Cd(HL)(dmbpy)]·DMF}n(3)[bpea=bis(4-pyridyl)ethane; bpp=1,3-bis(4-pyridyl)propane; dmbpy=5,5′-dimethyl-2,2′-bipyridine]. 3个配合物分别表现出有趣的2D→2D穿插结构和一维带状结构. 荧光性质测试结果表明, 所有配合物的荧光均可被Cr2O72?猝灭, 而在乙酰丙酮的DMF溶液中, 只有配合物1表现出明显的荧光增强. 羧酸配体的柔性、 含氮配体的类型和结构可以调控配合物的结构和荧光性能.
中图分类号:
TrendMD:
咸国轩, 于玉娥, 陈玉倩, 万小雨, 王素娜, 卢静. 辅助配体调控的荧光Cd-MOFs: 乙酰丙酮的荧光增强和Cr(Ⅵ)的荧光猝灭. 高等学校化学学报, 2020, 41(12): 2725.
XIAN Guoxuan, YU Yu’e, CHEN Yuqian, WAN Xiaoyu, WANG Suna, LU Jing. Coligand Induced Luminescent Cd-MOFs: Luminescence Enhancement Toward Acetylacetone and Quenching Toward Cr2O72-. Chem. J. Chinese Universities, 2020, 41(12): 2725.
| Compd. | 1 | 2 | 3 |
|---|---|---|---|
| CCDC No. | 2003735 | 2003736 | 2003737 |
| Formula | C32H36N4O10Cd | C33H40N4O11Cd | C32H32N4O8Cd |
| Formula weight | 749.05 | 781.09 | 713.03 |
| T/K | 298 | 298 | 298 |
| Crystal system | Triclinic | Triclinic | Monoclinic |
| Space group | C2/c | ||
| a/nm | 1.02640(8) | 1.02059(10) | 3.5513(3) |
| b/nm | 1.07661(9) | 1.24810(12) | 0.87420(8) |
| c/nm | 1.58609(12) | 1.51020(15) | 1.96671(17) |
| α/(°) | 108.934(3) | 65.763(1) | 90 |
| β/(°) | 99.127(2) | 82.945(3) | 94.036(2) |
| γ/(°) | 93.770(1) | 77.905(2) | 90 |
| V/nm3 | 1.6239(2) | 1.7137(3) | 6.0906(9) |
| Z | 2 | 2 | 8 |
| Dc/(g·cm-3) | 1.532 | 1.514 | 1.555 |
| μ/mm-1 | 0.736 | 5.664 | 0.776 |
| θ range/(°) | 2.7—25.0 | 3.2—66.0 | 2.3—25.0 |
| Index range | -12≤h≤11, -8≤k≤12, -18≤l≤13 | -12≤h≤12, -14≤k≤9, -17≤l≤16 | -42≤h≤40, -8≤k≤10, -23≤l≤22 |
| R1, wR2 [I> 2σ(I)] | 0.0552, 0.1433 | 0.0612, 0.1192 | 0.0458, 0.1064 |
| Goodness?of?fit on F2 | 0.990 | 0.976 | 1.006 |
Table 1 Crystallographic data and structure refinement parameters for complexes 1—3
| Compd. | 1 | 2 | 3 |
|---|---|---|---|
| CCDC No. | 2003735 | 2003736 | 2003737 |
| Formula | C32H36N4O10Cd | C33H40N4O11Cd | C32H32N4O8Cd |
| Formula weight | 749.05 | 781.09 | 713.03 |
| T/K | 298 | 298 | 298 |
| Crystal system | Triclinic | Triclinic | Monoclinic |
| Space group | C2/c | ||
| a/nm | 1.02640(8) | 1.02059(10) | 3.5513(3) |
| b/nm | 1.07661(9) | 1.24810(12) | 0.87420(8) |
| c/nm | 1.58609(12) | 1.51020(15) | 1.96671(17) |
| α/(°) | 108.934(3) | 65.763(1) | 90 |
| β/(°) | 99.127(2) | 82.945(3) | 94.036(2) |
| γ/(°) | 93.770(1) | 77.905(2) | 90 |
| V/nm3 | 1.6239(2) | 1.7137(3) | 6.0906(9) |
| Z | 2 | 2 | 8 |
| Dc/(g·cm-3) | 1.532 | 1.514 | 1.555 |
| μ/mm-1 | 0.736 | 5.664 | 0.776 |
| θ range/(°) | 2.7—25.0 | 3.2—66.0 | 2.3—25.0 |
| Index range | -12≤h≤11, -8≤k≤12, -18≤l≤13 | -12≤h≤12, -14≤k≤9, -17≤l≤16 | -42≤h≤40, -8≤k≤10, -23≤l≤22 |
| R1, wR2 [I> 2σ(I)] | 0.0552, 0.1433 | 0.0612, 0.1192 | 0.0458, 0.1064 |
| Goodness?of?fit on F2 | 0.990 | 0.976 | 1.006 |
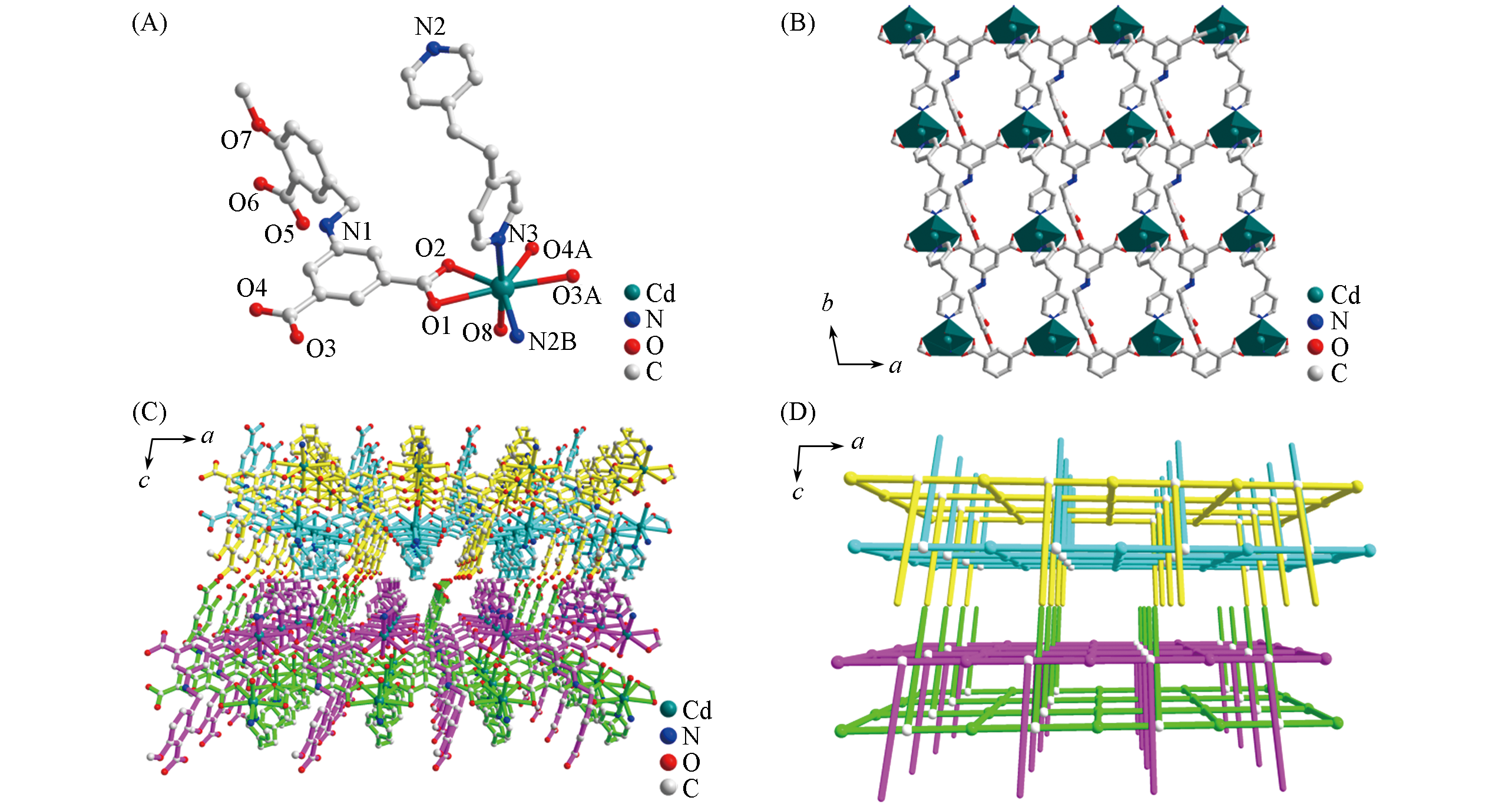
Fig.1 Assymetric unit(A), 2D layer along ab plane(B), perspective view(C) and simplified 3D supramolecular strcture(D) of complex 1(A) Symmetry codes: A. ?1+x, y, z; B. x, 1+y, z.
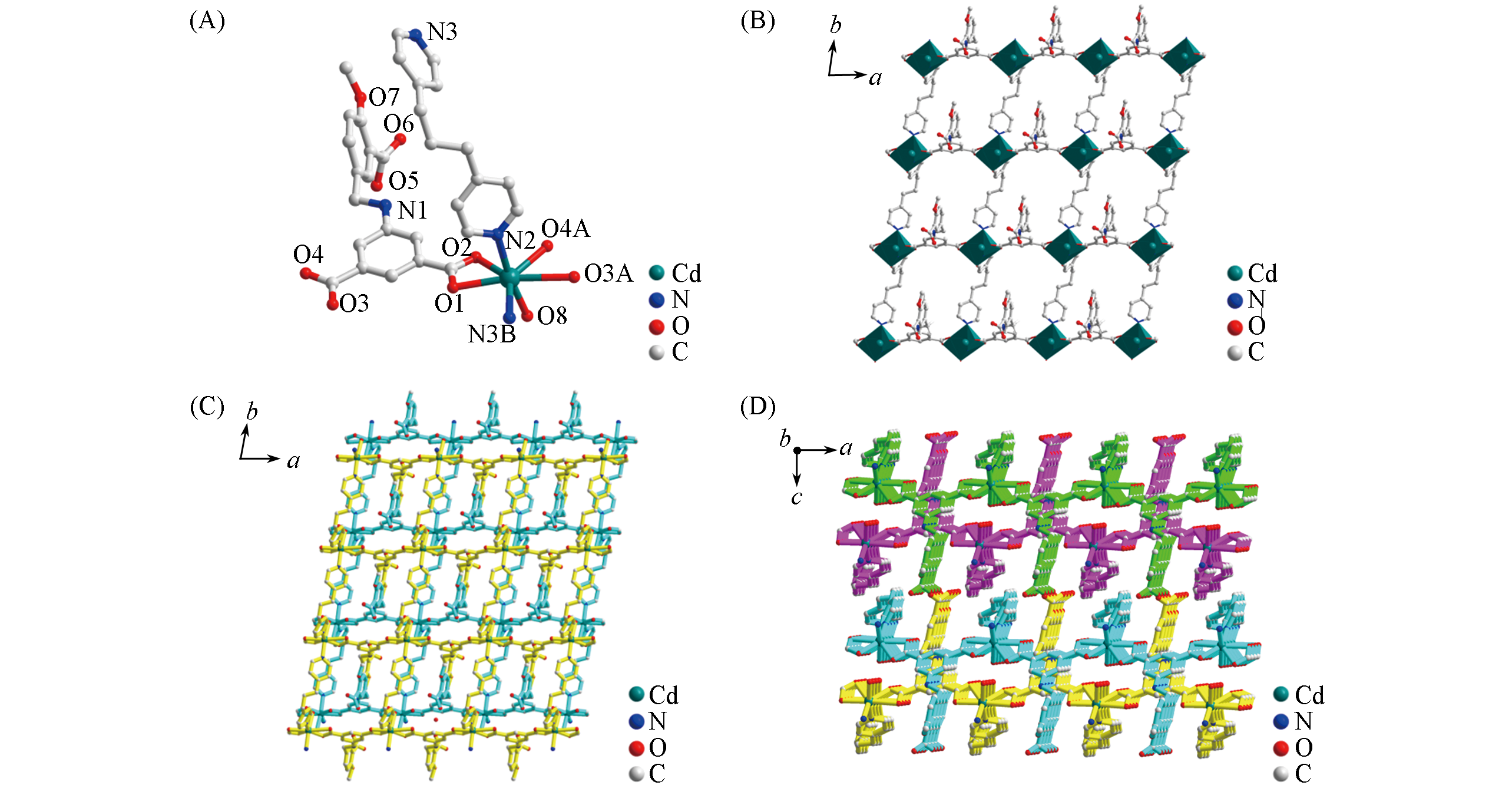
Fig.2 Assymetric unit(A), 2D layer along ab plane(B) and perspective views of 3D supramolecular structure along c(C) and b(D) axis of complex 2Symmetry codes: A. ?1+x, y, z; B. x, 1+y, z.
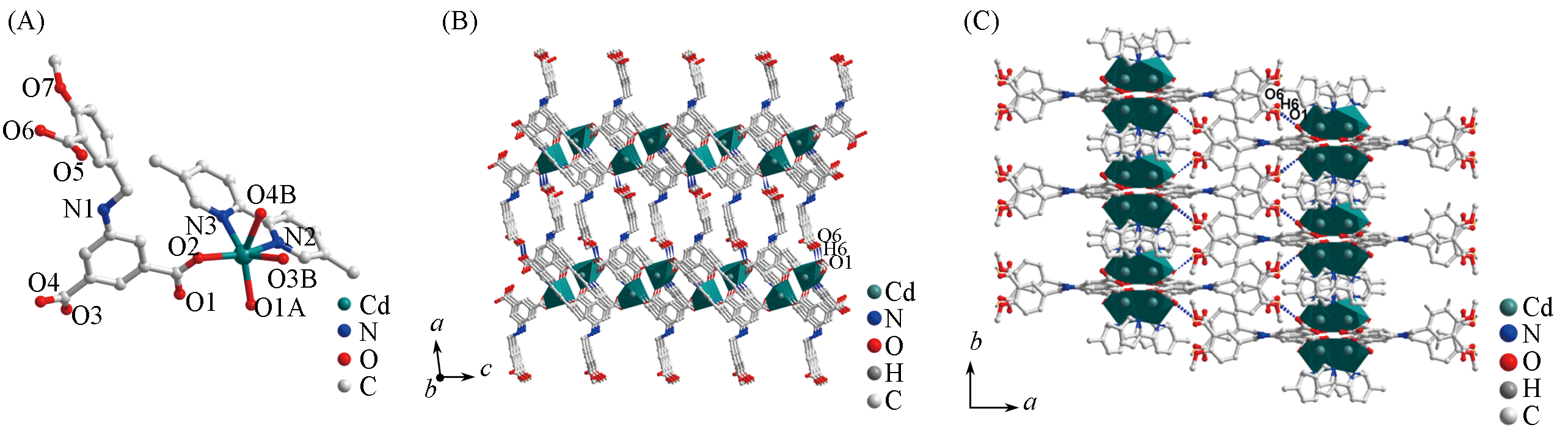
Fig.3 Assymetric unit(A), 1D band structure along ac plane(B) and perspective view of 3D supramolecular structure along c axis(C) of complex 3Symmetry codes: A. 1-x, y, 0.5-z; B. x, 2-y, 0.5+z.
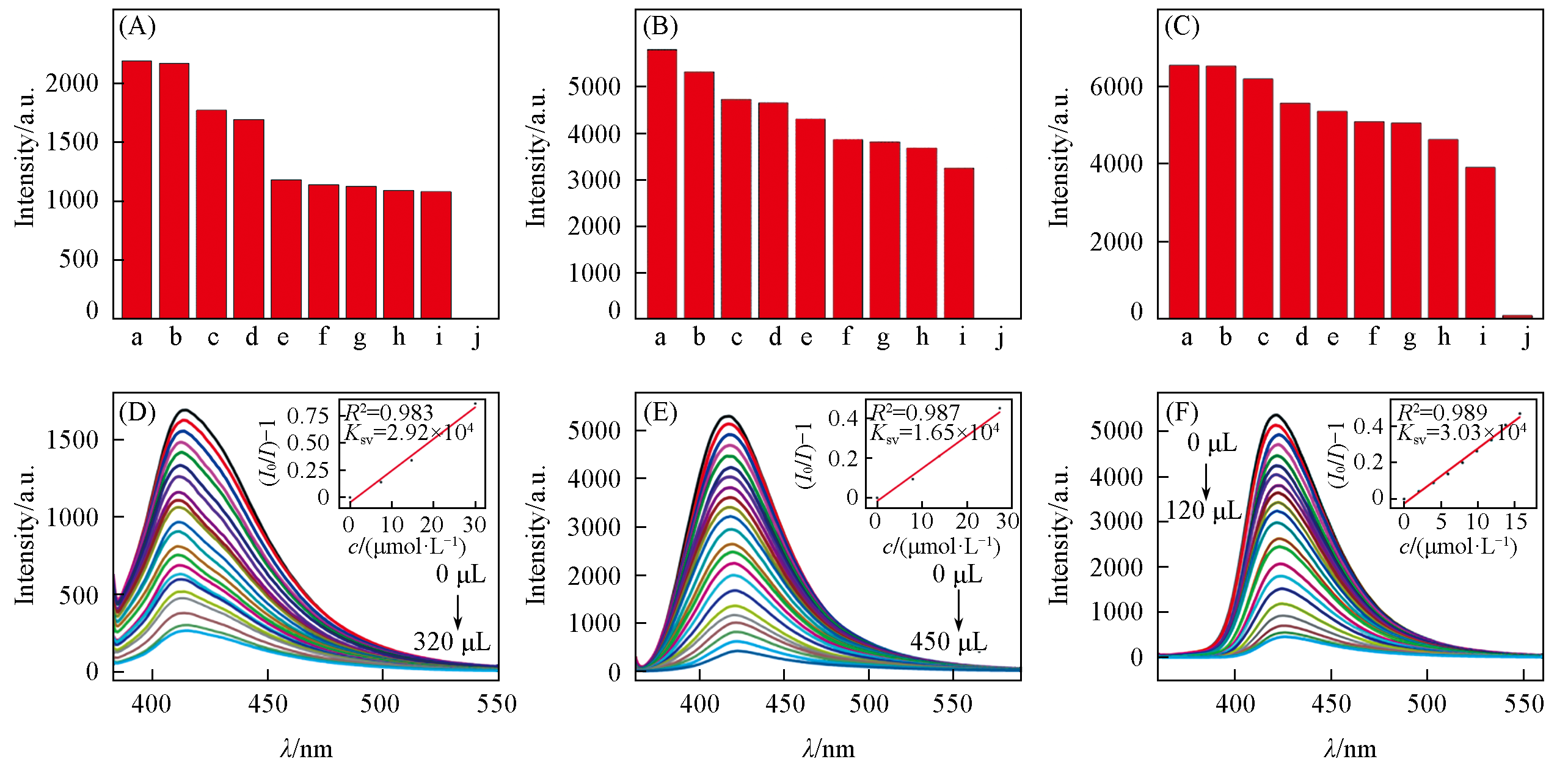
Fig.6 Emission intensities in DMF with different anions(1×10-3 mol/L, A—C) and luminescence titration curves toward Cr2O72-(D—F) of complexes 1(A, D), 2(B, E), 3(C, F)Insets of (D)—(F) are relative S-V fitting curves. (A) a. Br-; b. SO42-; c. Cl-; d. DMF; e. NO-3; f. C2O42-; g. CO32-; h. OH-; i. F-; j. Cr2O72-; (B) a. Cl-; b. DMF; c. Br-; d. C2O42-; e. NO-3; f. F-; g. SO42-; h. CO32-; i. OH-; j. Cr2O72-; (C) a. F-; b. Cl-; c. CO32-; d. SO42-; e. C2O42-; f. DMF; g. Br-; h. OH-; i. NO-3; j. Cr2O72-.
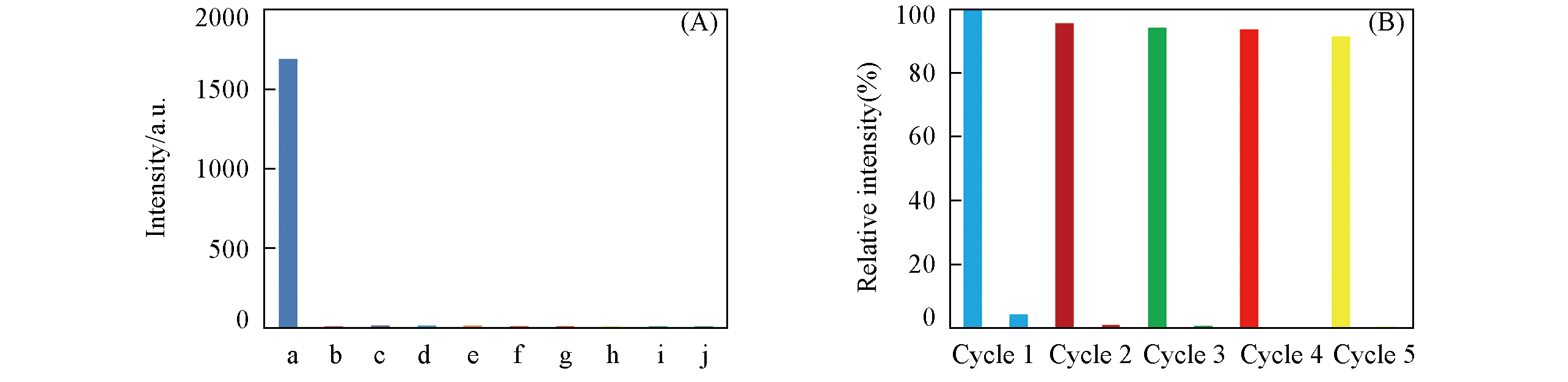
Fig.7 Interference experiment results of complex 1 in the suspensions of different anions with and without Cr2O72-, respectively(A) and recycling luminescence intensity of complex 1 after five runs(B)(A) a. DMF; b. Cr2O72?; c. Cr2O72?+Br-; d. Cr2O72?+NO3?; e. Cr2O72?+CO32?; f. Cr2O72?+F?; g. Cr2O72?+OH?; h. Cr2O72?+SO42?; i. Cr2O72?+C2O42?; j. Cr2O72?+Cl?. (B) In each cycle, the high and low columns represent the relative luminescence intensity before and after sensing Cr2O72? compared with the original blank sample, respectively.
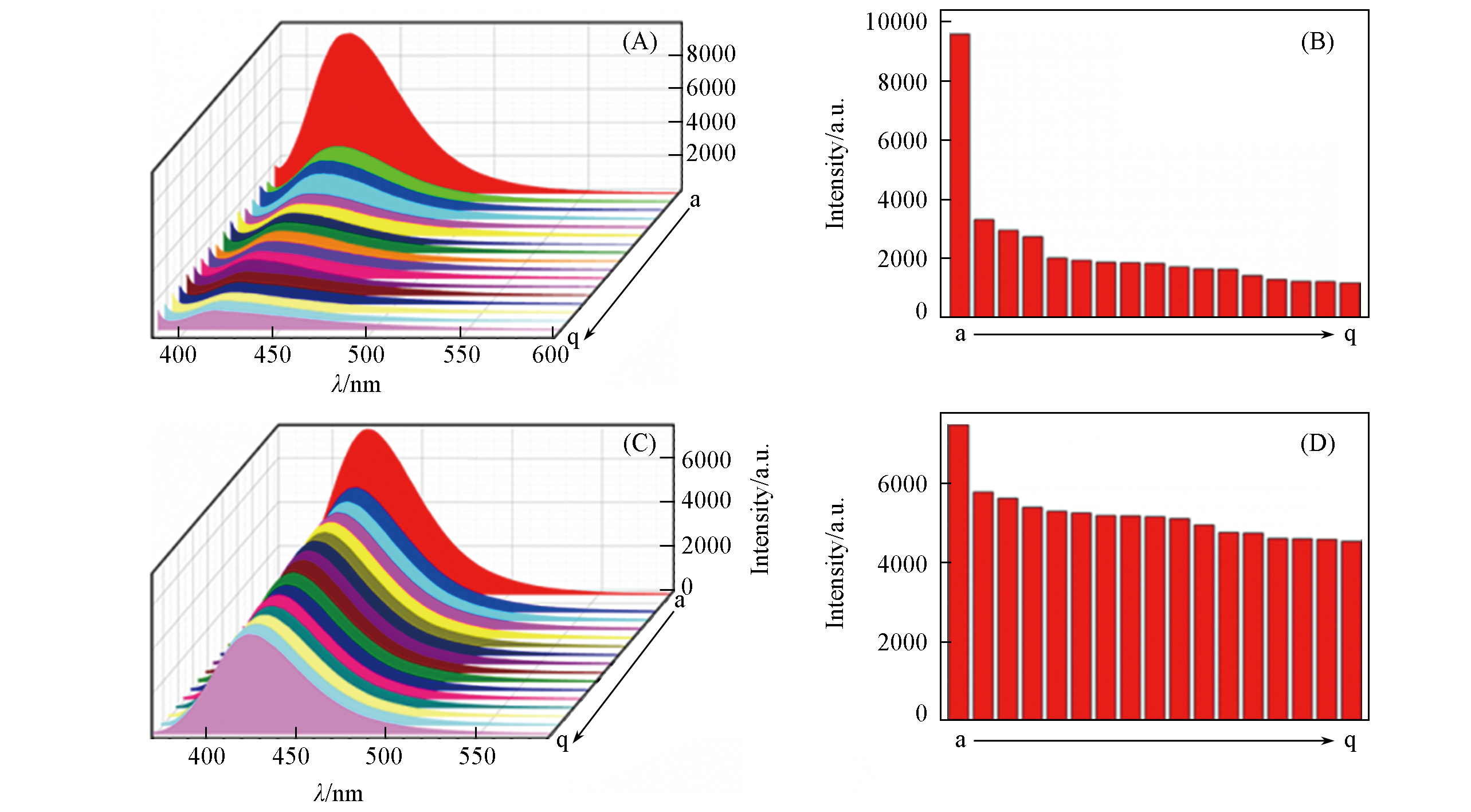
Fig.8 Luminescence spectra(A, C) and intensities(B, D) of complex 1(A, B) and complex 2(C, D) in DMF with different organic molecules(1×10-3 mol/L)(A, B) a. acac; b. 1-butanol; c. 1-propanol; d. isopropanol; e. DMA; f. CH2Cl2; g. 1-octanol; h. acetone; i. isobutanol; j. DMSO; k.CH2CN; L. DMF; m. CH3OH; n. CHCl3; o. THF; p. 1-OCTANOL; q. EtOH.(C, D) a. acac; b. CH3CN; c. isopropanol; d. 1-butanol; e. CH3OH; f. THF; g. DMA; h. EtOH; i. acetone; j. 1-octanol; k. CH2Cl; l. 1-propanol; m. DMF; n. isobutanol; o. DMSO; p. CHCl3; q. isooctanol.
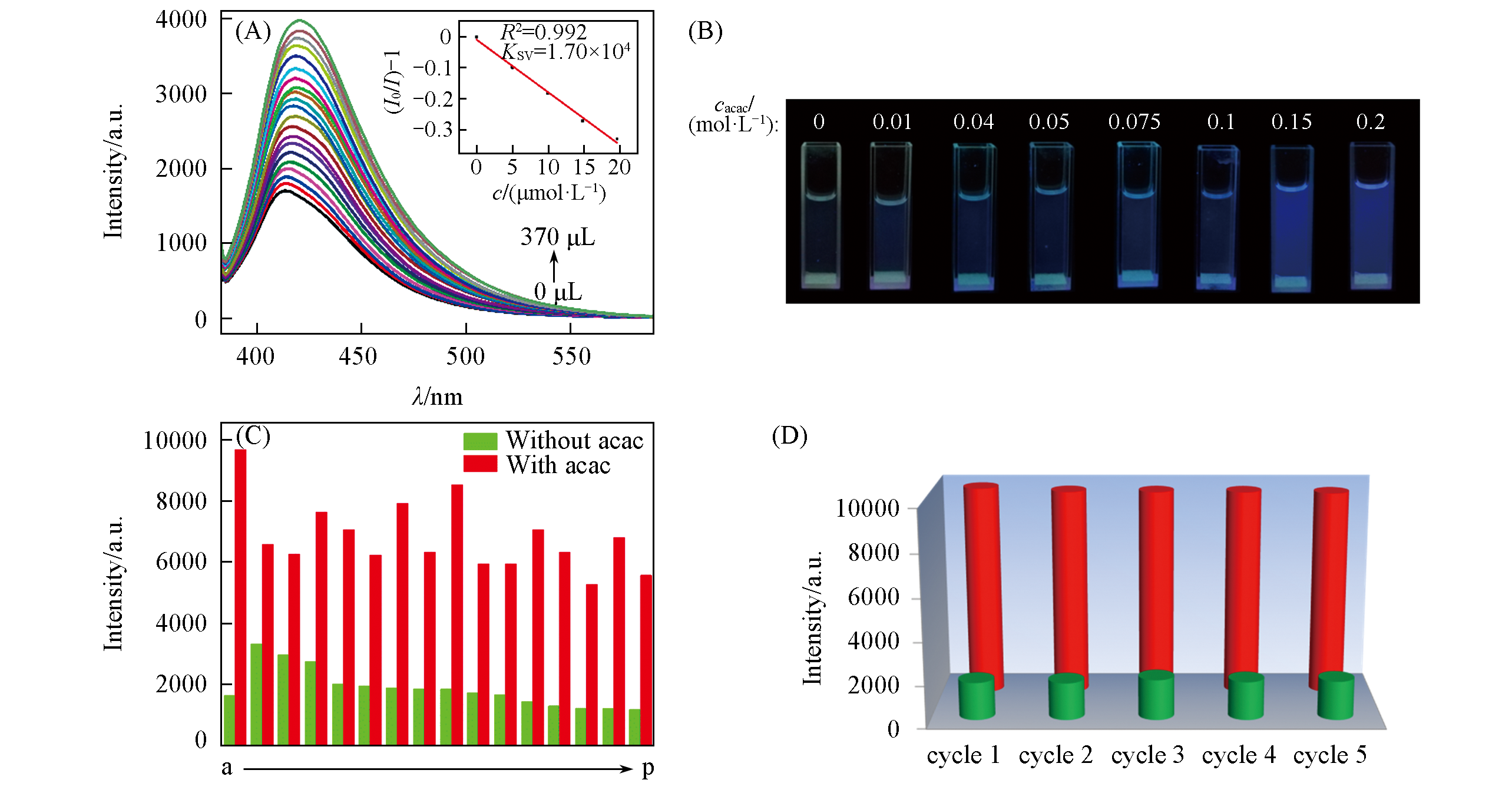
Fig.9 Luminescence titration curves of complex 1 after addition of different amounts of acac in DMF(1×10-3 mol/L)(A), images of complex 1 with different concentrations of acac in DMF under 365 nm UV light(B), interference experiment results of complex 1 in the suspensions of different anions with and without acac, respectively(C) and recycling luminescence intensity of complex 1 after five runs(D)(C) a. Blank; b. n-butanol; c. 1-propanol; d. isopropanol; e. DMA; f. CH2Cl2; g. 1-octanol; h. acetone; i. isobutanol; j. DMSO; k. CH3CN; l. CH3OH; m. CHCl3; n. THF; o. 1-octanol; p. EtOH. (D) Green and red columns represent the luminescence intensity before and after sensing acac, respectively.
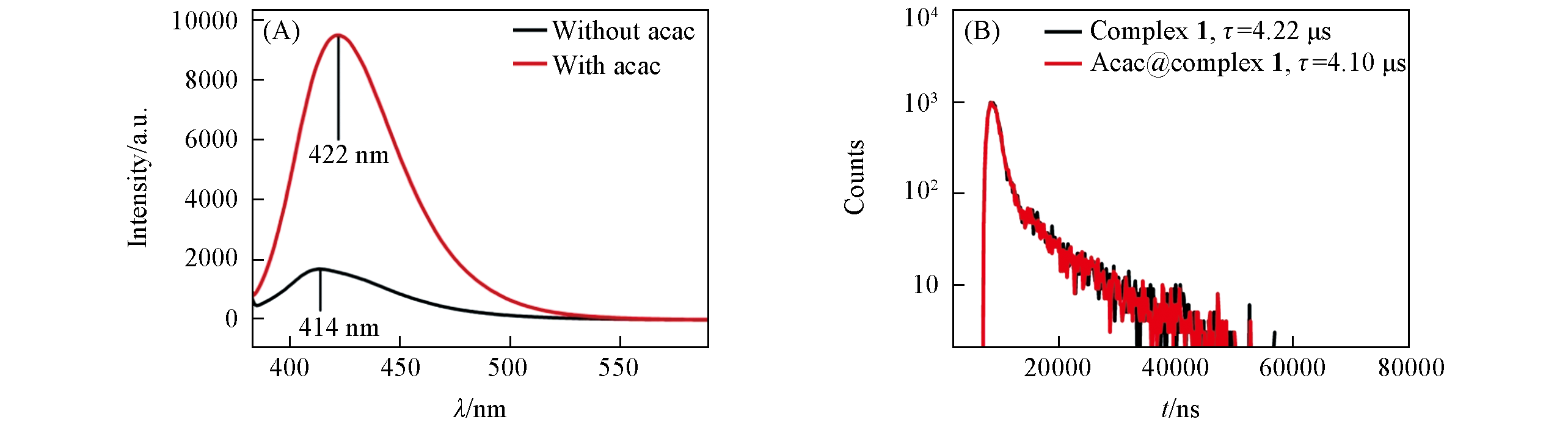
Fig.10 Luminescence spectra of complex 1 with and without acac in DMF(A) and luminescence lifetime of complex 1 at solid state before and after treatment with acac(1×10-3 mol/L)(B)
| 38 | Buragohain A., Yousufuddin M., Sarma M., Biswas S., Cryst. Growth. Des., 2016, 16, 842—851 |
| 39 | Zhao Y., Xu X. Y.,Qiu L., Kang X. J., Wen L. L., Zhang B. G., ACS Appl. Mater. Interfaces., 2017, 9, 15164—15175 |
| 40 | Lin Y. N., Zhang X. P.,Chen W. J., Shi W., Cheng P., Inorg. Chem., 2017, 56, 11768—11778 |
| 41 | Parmar B., Rachuri Y., Bisht K. K., Laiya R., Suresh E., Inorg. Chem., 2017, 56, 2627—2638 |
| 42 | Chen S. G., Shi Z. Z., Qin L., Jia H. L., Zheng H. G., Cryst. Growth. Des., 2017, 17, 67—72 |
| 43 | Zhang X. L., Zhan Z. Y., Liang X. Y., Chen C., Liu X. L., Jia Y. J., Hu M., Dalton Trans., 2018, 47, 3272—3282 |
| 44 | Liu D. M., Su Y. J., Li S. S., Li J. J., Xu Q. W., Li X., Chem. J. Chinese Universities, 2020, 41(2), 253—261(刘东枚, 苏雅静, 李珊珊, 李佳佳, 许奇炜, 李夏. 高等学校化学学报, 2020, 41(2), 253—261) |
| 45 | Pramanik S., Zheng C., Zhang X., Emge T. J., Li J., J. Am. Chem. Soc., 2011, 133, 4153—4155 |
| 1 | Zhitkovich A., Chem. Res. Toxicol., 2005, 18, 3—11 |
| 2 | Thompson C. M., Kirman C. R., Proctor D. M., Haws L. C., Suh M., Hays S. M., Gregory J. H., Harris M. A., J. Appl. Toxicol., 2014, 34, 525—536 |
| 3 | US Department of Health and Human Services, Toxicological Profile for Chromium, Public Health Service Agency for Toxic Substances and Diseases Registry, Washington DC, 2005 |
| 4 | Zeng H. Y., Duan Z. K., Chen H. Z., Zeng Z. D., Luo A. W., Chin. J. Anal. Chem., 2011, 3, 382—386 |
| 5 | Zhou L. L., Li C., Weng X. C., Magn. Reson. Chem., 2016, 54, 222—226 |
| 6 | Reyes Z., SilversteinR. M., J. Am. Chem. Soc., 1958, 80, 6367—6372 |
| 7 | Reeves L. W., Allan E. A.,Strømme K. O., Can. J. Chem., 1960, 38, 1249—1254 |
| 8 | Hu Z., Deibert B. J., Li J., Chem. Soc. Rev., 2014, 43, 5815—5840 |
| 9 | Silva P., Vilela S. M. F., Tomé J. P. C., Almeida P. F. A., Chem. Soc. Rev., 2015, 44, 6774—6803 |
| 10 | Lustig W. P., Mukherjee S., Rudd N. D., Desai A. V., Li J., Ghosh S. K., Chem. Soc. Rev., 2017, 46, 3242—3285 |
| 11 | Zhang Y., Yuan S., Day G., Wang X., Yang X., Zhou H. C., Coord. Chem. Rev., 2018, 354, 28—45 |
| 12 | He J., Xu J. L., Yin J. C., Li N., Bu X. H., Sci. China Mater., 2019, 62, 1655—1678 |
| 13 | Yan B., J. Mater. Chem. C, 2019, 7, 8155—8175 |
| 14 | Yang H., Wang F., Tan Y. X., Kang Y., Li T. H., Zhang J., Chem. Asian J., 2012, 7, 1069—1073 |
| 15 | Kang X. M., Cheng R. R., Xu H., Wang W. M., Zhao B., Chem. Eur. J., 2017, 23, 13289—13293 |
| 16 | Zou J. Y., Li L., You S. Y., Chen K. H., Dong X. N., Chen Y. H., Cui J. Z., Cryst. Growth. Des., 2018, 18, 3997—4003 |
| 17 | Kang X. M., Fan X. Y., Hao P. Y., Wang W. M., Zhao B., Inorg. Chem. Front., 2019, 6, 271—277 |
| 18 | Li L. J., Zou Y., You S. Y., Liu Y. W., Cui H. M., Zhang S. W., Dyes and Pigments, 2020, 173, 108004 |
| 19 | Xiao Q. Q., Dong G. Y., Li Y. H., Cui G. H., Inorg. Chem., 2019, 58, 15696—15699 |
| 20 | Shi Y. S., Li Y. H., Cui G. H., Dong G. Y., CrystEngComm, 2020, 22, 905—914 |
| 21 | Yang Y. J., Li Y. H., Liu D. L., Cui G. H., CrystEngComm, 2020, 22, 1166—1175 |
| 22 | Li A. L., Qu Y. H., Fu L. S., Han C., Cui G. H., CrystEngComm, 2020, 22, 2656—2666 |
| 23 | Yao S. L., Liu S. J., Tian X. M., Zheng T. F., Cao C., Niu C. Y., Chen Y. Q., Chen J. L., Huang H. P., Wen H. R., Inorg. Chem., 2019, 58, 3578—3581 |
| 24 | Wang S. N., Cao T. T., Yan H.,Li Y. W., Lu J., Ma R. R., Li D. C., Dou J. M., Bai J. F.,Inorg. Chem., 2016, 55, 5139—5151 |
| 25 | Wang S. N., Ma R. R., Chen Z. W., Li Y. W., Cao T. T., Zhou C. H., Bai J. F., Sci. China Chem., 2016, 59, 948—958 |
| 26 | Chen Z. W., Mi X. N., Lu J., Wang S. N., Li Y. W., Dou J. M., Li D. C., Dalton Trans., 2018, 47, 6240—6249 |
| 27 | Mi X. N., Sheng D. F., Yu Y. E., Wang Y. H., Zhao L. M., Lu J., Li Y. W., Li D. C., Dou J. M., Duan J. G., Wang S. N., ACS Appl. Mater. Interfaces, 2019, 11, 7914—7926 |
| 28 | Yu Y. E., Wang Y. H., Yan H., Lu J., Liu H. T., Li Y. W., Wang S. N., Li D. C., Dou J. M., Yang L., Zhou Z., Inorg. Chem., 2020, 59, 3828—3837 |
| 29 | Yu Y. E., Wang Y. H., Xu H. J., Lu J., Wang H. W., Li D. C., Dou J. M., Li Y. W., Wang S. N., CrystEngComm, 2020, 22, 3759—3767 |
| 30 | Chen Z. W., Mi X. N., Wang Y. H., Yu Y. E., Wang S. N., Journal of Liaocheng University(Nat. Sci.) 2018, 31(4), 83—93(陈志伟, 宓秀娜, 王昱皓, 于玉娥, 王素娜. 聊城大学学报(自然科学版), 2018, 31(4), 83—93) |
| 46 | Pramanik S., Hu Z., Zhang X., Zheng C., Kelly S., Li J., Chem. Eur. J., 2013, 19, 15964—15971 |
| 47 | Wang F., Dong C., Wang Z., Cui Y., Wang C., Zhao Y., Li G., Eur. J. Inorg. Chem., 2014, 6239—6245 |
| 31 | Yang L., He C., Liu X., Zhang J., Sun H., Guo H. M., Chem. Eur. J., 2016, 22, 5253—5260 |
| 32 | Bruker S., SAINT(Version 6.02), SHELXTL(Version 6.10) and SADABS(Version 2.03), Bruker AXS Inc, Madison, Wisconsin, 2002 |
| 33 | Sheldrick G. M., A Program for the Siemens Area Detector ABSorption Correction, University of Göttingen, Göttingen, 1997 |
| 34 | Sheldrick G. M., SHELXL⁃14, Program for the Solution of Crystal Structure, University of Göttingen, Göttingen, 1997 |
| 35 | Sheldrick G. M., SHELXL⁃14, Program for Crystal Structure Refinement, University of Göttingen, Göttingen, 1997 |
| 36 | Spek A. L., J. Appl. Cryst., 2003, 36, 7 |
| 37 | Wenger O. S., Chem. Rev., 2013, 113, 3686—3733 |
| [1] | 马鉴新, 刘晓东, 徐娜, 刘国成, 王秀丽. 一种具有发光传感、 安培传感和染料吸附性能的多功能Zn(II)配位聚合物[J]. 高等学校化学学报, 2022, 43(1): 20210585. |
| [2] | 李占峰, 刘本学, 刘晓婵, 王新强, 张晶, 于诗摩, 赵新富, 张新恩, 伊希斌. 氧化锆湿凝胶中乙酰丙酮配体的脱除机理及气凝胶复合材料的制备[J]. 高等学校化学学报, 2021, 42(9): 2904. |
| [3] | 李然, 张旭东, 穆丽丹, 孙童, 艾刚刚, 沙夜龙, 张玉琦, 王记江. 三联噻吩衍生物功能化SiO2反蛋白石光子晶体荧光薄膜的制备及应用[J]. 高等学校化学学报, 2021, 42(9): 2989. |
| [4] | 刘东枚,苏雅静,李姗姗,许奇炜,李夏. 4-(4-羧基苯氧基)间苯二甲酸构筑的过渡金属配位聚合物: 合成、 晶体结构、 荧光传感与光催化[J]. 高等学校化学学报, 2020, 41(2): 253. |
| [5] | 阿丽, 王勇. 多肽荧光传感器检测铜离子[J]. 高等学校化学学报, 2020, 41(12): 2736. |
| [6] | 周思慧, 李琼, 张婷, 庞代文, 唐宏武. 基于碳点的荧光纳米开关灵敏检测Cu(Ⅱ)离子和焦磷酸盐[J]. 高等学校化学学报, 2019, 40(8): 1593. |
| [7] | 马玉坤, 王海君, 郭梦岩. 狼毒乙素分子印迹膜荧光传感器的制备及在中药材检测中的应用[J]. 高等学校化学学报, 2019, 40(7): 1381. |
| [8] | 石宝珍,李杉,王佃鹏,周云志,孙锦玉. Co(Ⅱ)-Zn(Ⅱ)掺杂配位聚合物的合成及物理性能[J]. 高等学校化学学报, 2019, 40(12): 2443. |
| [9] | 张春燕, 罗建新, 李文军, 欧丽娟, 喻桂朋, 潘春跃. 单分散键合型含铕聚苯乙烯微球的制备与荧光传感性能[J]. 高等学校化学学报, 2019, 40(1): 153. |
| [10] | 王冬梅, 刘子华, 李光华, 刘云凌, 李春霞. 铟基双金属配位聚合物的合成、 结构及荧光性质[J]. 高等学校化学学报, 2018, 39(9): 1886. |
| [11] | 宋伟, 王力群, 曾双利, 王莉, 范勇, 徐家宁. 镉配位聚合物的原位水热合成、晶体结构及荧光性质[J]. 高等学校化学学报, 2018, 39(7): 1406. |
| [12] | 刘丽丽, 台夕市, 刘均波, 李丹, 周小晶, 张丽君, 危潇飞. 双活性中心催化剂的制备及催化合成炔丙基胺[J]. 高等学校化学学报, 2018, 39(3): 482. |
| [13] | 李建, 谢林霞, 梁足培, 国荣, 刘晨昱, 马淑兰. 四硫代钼酸根/1-辛烷磺酸根/LEuH复合体发光性能及对Hg2+的识别[J]. 高等学校化学学报, 2018, 39(10): 2154. |
| [14] | 战岩, 俎鸿儒, 黄棣, 刘应亮, 胡超凡. 溶剂热插层法制备荧光石墨相氮化碳量子点及其在Fe3+检测中的应用[J]. 高等学校化学学报, 2017, 38(9): 1556. |
| [15] | 杨昕, 张迪, 宋立敏, 许茜, 许卉, 刘珂. 基于光谱分析的姜黄素碱性自氧化及其产物的抗氧化活性[J]. 高等学校化学学报, 2017, 38(9): 1549. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||