

高等学校化学学报 ›› 2017, Vol. 38 ›› Issue (1): 115.doi: 10.7503/cjcu20160411
收稿日期:2016-06-07
出版日期:2017-01-10
发布日期:2016-12-15
作者简介:联系人简介: 李孔斋, 男, 博士, 教授, 主要从事能源催化方面的研究. E-mail: 基金资助:
ZENG Liangpeng1, HUANG Fan2, ZHU Xing1, ZHENG Min1, LI Kongzhai1,*( )
)
Received:2016-06-07
Online:2017-01-10
Published:2016-12-15
Contact:
LI Kongzhai
E-mail:kongzhai.li@aliyun.com
Supported by:摘要:
采用水热法制备了Co3O4/CeO2(x)[x为钴铈原子摩尔比n(Co):n(Ce)=6:49:1]和Ce1-yCoyO2-δ(y=0.10.4)2个系列复合氧化物, 并表征了材料的物理化学性质, 考察了这些氧化物作为氧载体参与甲烷化学链转化(化学链燃烧和化学链部分氧化)的反应性能. 结果表明, 2类复合氧化物的甲烷反应活性均明显优于单一氧化物CeO2或Co3O4, 但2类氧载体上的甲烷反应产物的选择性具有明显差异. Ce1-yCoyO2-δ氧载体形成了Ce-Co-O固溶体, 储氧能力明显增强, 体相晶格氧迁移速率与甲烷活化速率匹配较好, 甲烷反应产物以CO和H2的合成气为主, 有利于甲烷的化学链部分氧化. Co3O4/CeO2(x)氧载体中CeO2与Co3O4之间的相互作用改善了材料的储氧能力和氧化活性, 其与甲烷反应时主要生成CO2, 有利于甲烷化学链燃烧. 连续性化学链循环实验表明, 2类氧载体均具有较好的再生性能和循环稳定性.
中图分类号:
TrendMD:
曾良鹏, 黄樊, 祝星, 郑敏, 李孔斋. 铈基与钴基Co3O4-CeO2氧载体上甲烷化学链转化特性: 产物选择性控制. 高等学校化学学报, 2017, 38(1): 115.
ZENG Liangpeng, HUANG Fan, ZHU Xing, ZHENG Min, LI Kongzhai. Chemical Looping Conversion of Methane over CeO2-based and Co3O4-based Co3O4-CeO2 Oxygen Carriers:Controlling of Product Selectivity†. Chem. J. Chinese Universities, 2017, 38(1): 115.
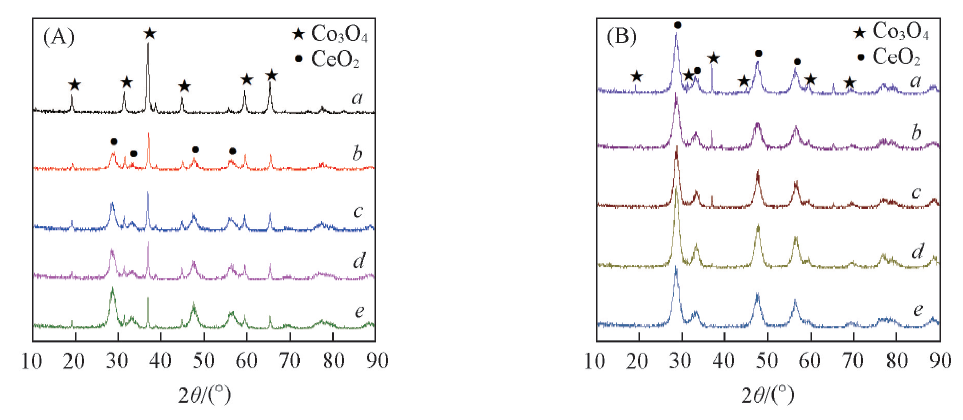
Fig.3 XRD patterns of different Co3O4/CeO2(x)(A) and Ce1-yCoyO2-δ(B) oxygen carriers(A) a. Co3O4; b. Co3O4/CeO2(9:1); c. Co3O4/CeO2(8:2); d. Co3O4/CeO2(7:3); e. Co3O4/CeO2(6:4).(B) a. Ce0.6Co0.4O2-δ; b. Ce0.7Co0.3O2-δ; c. Ce0.8Co0.2O2-δ; d. Ce0.9Co0.1O2-δ; e. CeO2.
| Oxygen carrier | SBET/(m2·g-1) | Lattice constant/nm | |
|---|---|---|---|
| Co3O4 | CeO2 | ||
| Co3O4 | 39.30 | 0.5681 | |
| Co3O4/CeO2(9:1) | 35.57 | 0.5662 | 0.5410 |
| Co3O4/CeO2(8:2) | 31.22 | 0.5679 | 0.5410 |
| Co3O4/CeO2(7:3) | 24.30 | 0.5679 | 0.5417 |
| Co3O4/CeO2(6:4) | 34.45 | 0.5681 | 0.5416 |
| Ce0.6Co0.4O2-δ | 40.94 | 0.5686 | 0.5410 |
| Ce0.7Co0.3O2-δ | 42.84 | 0.4224 | 0.5406 |
| Ce0.8Co0.2O2-δ | 43.60 | 0.4219 | 0.5404 |
| Ce0.9Co0.1O2-δ | 58.29 | 0.4670 | 0.5404 |
| CeO2 | 131.06 | | 0.5419 |
Table 1 Specific surface areas and lattice constants of Co3O4 and CeO2 phases detected in the CeO2-Co3O4 oxygen carriers
| Oxygen carrier | SBET/(m2·g-1) | Lattice constant/nm | |
|---|---|---|---|
| Co3O4 | CeO2 | ||
| Co3O4 | 39.30 | 0.5681 | |
| Co3O4/CeO2(9:1) | 35.57 | 0.5662 | 0.5410 |
| Co3O4/CeO2(8:2) | 31.22 | 0.5679 | 0.5410 |
| Co3O4/CeO2(7:3) | 24.30 | 0.5679 | 0.5417 |
| Co3O4/CeO2(6:4) | 34.45 | 0.5681 | 0.5416 |
| Ce0.6Co0.4O2-δ | 40.94 | 0.5686 | 0.5410 |
| Ce0.7Co0.3O2-δ | 42.84 | 0.4224 | 0.5406 |
| Ce0.8Co0.2O2-δ | 43.60 | 0.4219 | 0.5404 |
| Ce0.9Co0.1O2-δ | 58.29 | 0.4670 | 0.5404 |
| CeO2 | 131.06 | | 0.5419 |
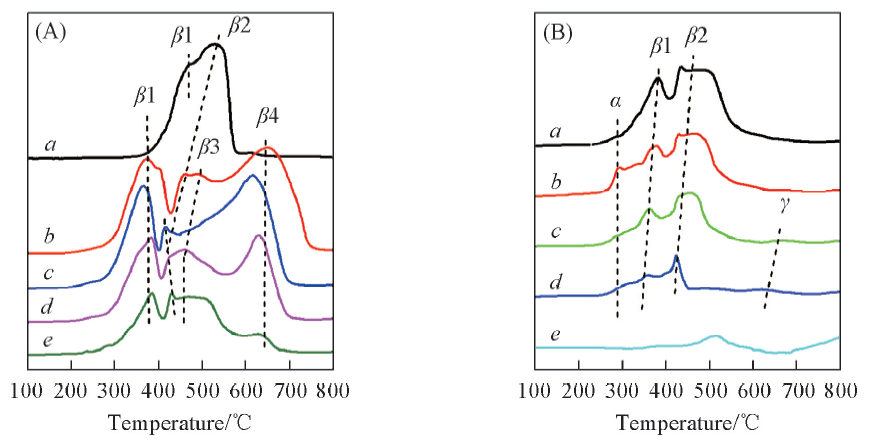
Fig.4 H2-TPR profiles of Co3O4/CeO2(x)(A) and Ce1-yCoyO2-δ(B) oxygen carriers with different Co/Ce molar ratios(A) a. Co3O4; b. Co3O4/CeO2(9:1); c. Co3O4/CeO2(8:2); d. Co3O4/CeO2(7:3); e. Co3O4/CeO2(6:4). (B) a. Ce0.6Co0.4O2-δ; b. Ce0.7Co0.3O2-δ; c. Ce0.8Co0.2O2-δ; d. Ce0.9Co0.1O2-δ; e. CeO2.
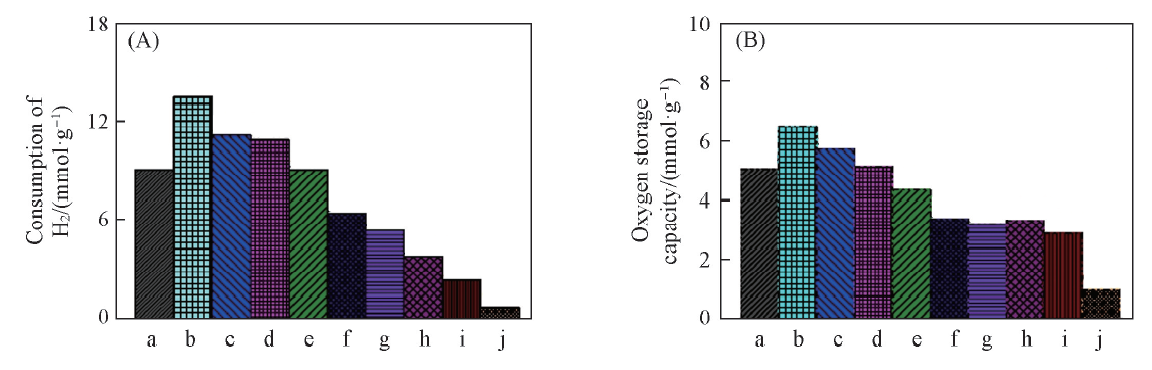
Fig.5 H2 consumption of H2-TPR test(A) and oxygen storage capacity(B) over different Co3O4/CeO2(x) and Ce1-yCoyO2-δoxygen carriersa. Co3O4; b. Co3O4/CeO2(9:1); c. Co3O4/CeO2(8:2); d. Co3O4/CeO2(7:3); e. Co3O4/CeO2(6:4); f. Ce0.6Co0.4O2-δ; g. Ce0.7Co0.3O2-δ; h. Ce0.8Co0.2O2-δ; i. Ce0.9Co0.1O2-δ; j. CeO2.
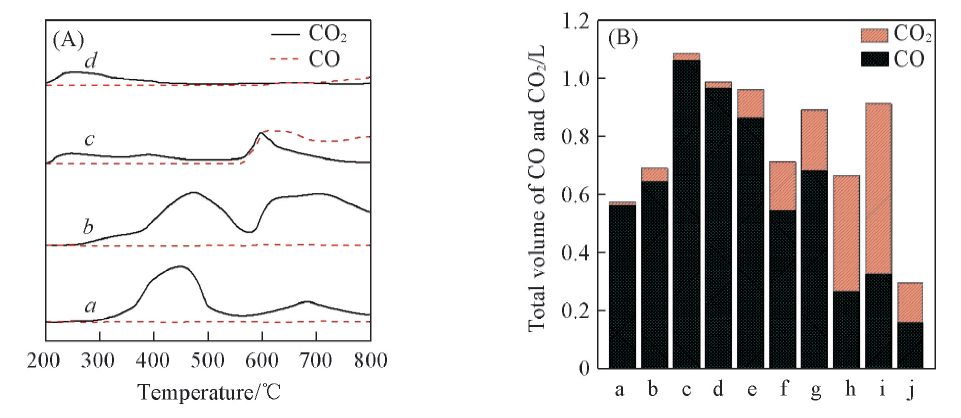
Fig.6 CH4-TPR profiles of Co3O4/CeO2(x) and Ce1-yCoyO2-δ oxygen carriers with different Co/Ce molar ratios(A) and the corresponding CO and CO2 average content bar charts of CH4-TPR test(B)(A) a. Co3O4; b. Co3O4/CeO2(8:2); c. Ce0.8Co0.2O2-δ; d. CeO2.(B) a. Co3O4; b. Co3O4/CeO2(9:1); c. Co3O4/CeO2(8:2); d. Co3O4/CeO2(7:3); e. Co3O4/CeO2(6:4); f. Ce0.6Co0.4O2-δ; g. Ce0.7Co0.3O2-δ; h. Ce0.8Co0.2O2-δ; i. Ce0.9Co0.1O2-δ; j. CeO2.
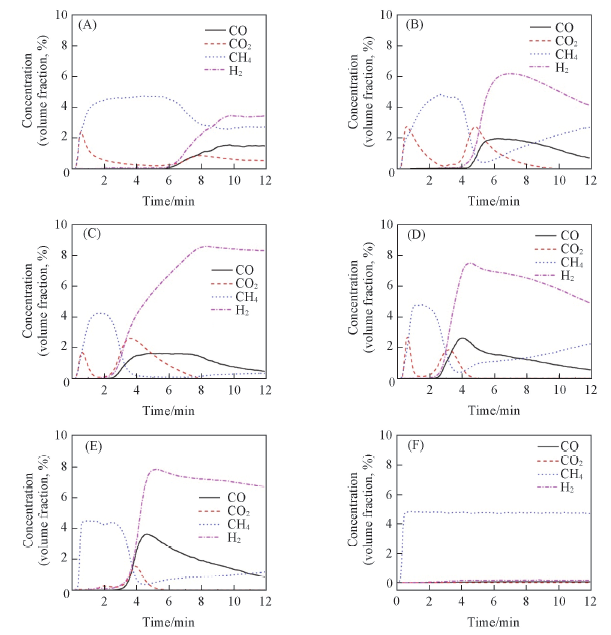
Fig.7 Evolution profiles of CO and CO2 for CH4 oxidation at 650 ℃ over Co3O4/CeO2(x) and Ce1-yCoyO2-δ oxygen carriers with different Co/Ce molar ratios(A) Co3O4; (B) Co3O4/CeO2(8:2); (C) Co3O4/CeO2(6:4); (D)Ce0.8Co0.2O2-δ; (E) Ce0.9Co0.1O2-δ; (F) CeO2.
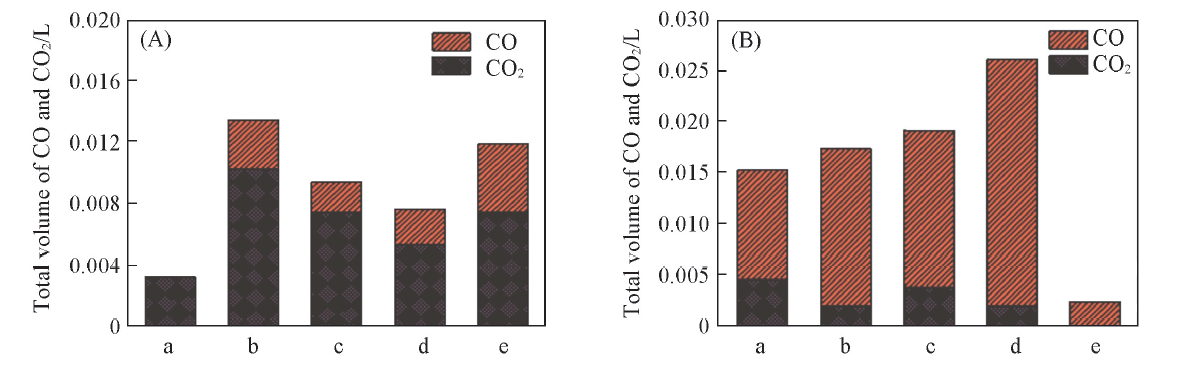
Fig.8 Corresponding CO and CO2 production on Co3O4/CeO2(x) in previous 6 min(A) and on Ce1-yCoyO2-δ in the whole reaction process(B) at 650 ℃(A) a. Co3O4; b. Co3O4/CeO2(9:1); c. Co3O4/CeO2(8:2); d. Co3O4/CeO2(7:3); e. Co3O4/CeO2(6:4); (B) a. Ce0.6Co0.4O2-δ; b. Ce0.7Co0.3O2-δ; c. Ce0.8Co0.2O2-δ; d. Ce0.9Co0.1O2-δ; e. CeO2.
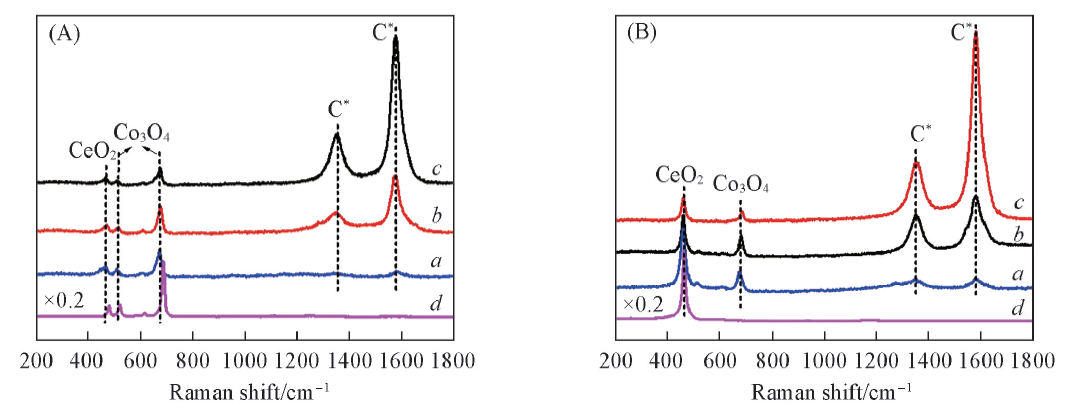
Fig.9 Raman spectra of Co3O4/CeO2(8:2)(A) and Ce0.8Co0.2O2-δ(B) after various reaction time with CH4 at 650 ℃Reaction time/min: a. 3; b. 5; c. 7. (A) d. Co3O4; (B) d. CeO2.
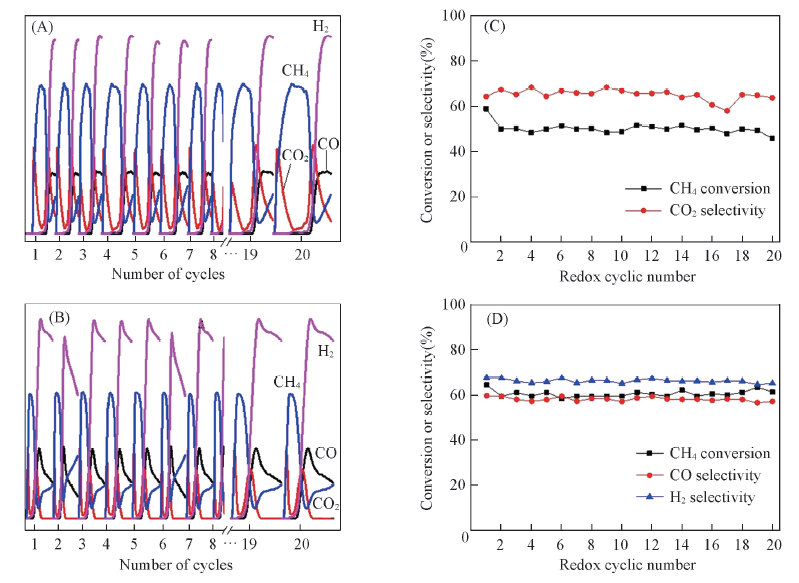
Fig.10 Transient responses of the cyclic reaction between CH4-Ar flow(10 min) and O2-Ar flow(20 min) over(A) Co3O4/CeO2(8:2) and(B) Ce0.8Co0.2O2-δ oxygen carrier at 650 ℃, and CH4 conversion or the selectivity of product gas over Co3O4/CeO2(8:2)(C), Ce0.8Co0.2O2-δ(D) as a function of redox cycle number
| [1] | Fernández J. R., Abanades J. C., Appl. Energy,2014, 135, 309—319 |
| [2] | Huang Z., He F., Zhao K., Zhen A. Q., Li H. B., Zhao Z. L., Pro. Chem., 2012, 24(8), 1599—1609 |
| (黄振, 何方, 赵坤, 郑安庆, 李海滨, 赵增立. 化学进展, 2012, 24(8), 1599—1609) | |
| [3] | Bolhàr-Nordenkampf J., Pröll T., Kolbitsch P., Hofbauer H., Energy Procedia,2009, 1(1), 19—25 |
| [4] | Leion H., Lyngfelt A., Mattisson T., Chem. Eng. Res. Des., 2009, 87(11), 1543—1550 |
| [5] | Qin W., Lin C. F., Cheng W. L., Xiao X. B., Chem. J. Chinese Universities,2016, 36(1), 116—123 |
| (覃吴, 林常枫, 程伟良, 肖显斌. 高等学校化学学报, 2016, 36(1), 116—123) | |
| [6] | Jin H. G., Wang B. Q., J. Eng. Thermophysics,2004, 25(2), 181—184 |
| (金红光, 王宝群. 工程热物理学报, 2004, 25(2), 181—184) | |
| [7] | Ma S. S. K., Chen L., Zhang S., Lin J., Jiang S. P., Int. J. Hydrogen Energy,2013, 38(30), 13300—13308 |
| [8] | Li K. Z., Wang H., Wei Y. G., Ao X. Q., Liu M. C., Pro. Chem., 2008, 20(9), 1306—1314 |
| (李孔斋, 王华, 魏永刚, 敖先权, 刘明春. 化学进展, 2008, 20(9), 1306—1314) | |
| [9] | Siriwardane R., Tian H. J., Richards G., Simonyi T., Poston J., Energy Fuels,2009, 23(8), 3885—3892 |
| [10] | Jin H., Toshihiro O. T., Ishida M., Ind. Eng. Chem. Res., 1999, 38(1), 126—132 |
| [11] | Mattisson T., Järdnäs A., Lyngfelt A., Energy Fuels,2003, 17(3), 643—651 |
| [12] | Qin W., Li Q., Dong C. Q., Cheng W. L., Yang Y. P., CIESC J., 2014, 65(8), 3136—3143 |
| (覃吴, 李渠, 董长青, 程伟良, 杨勇平. 化工学报, 2014, 65(8), 3136—3143) | |
| [13] | Ryu H. J., Jin G. T., Yi C. K., Greenhouse Gas Control Technologies,2005, 2(2), 1907—1910 |
| [14] | Adanez J., Abad A., Garcia-Labiano F., Gayan P., Diego L. F. D., Pro. Energy Combustion Sci., 2012, 38(2), 215—282 |
| [15] | Li Y., Shen W. J., Sci. Chin. Chem., 2012, 55(12), 2485—2496 |
| [16] | Otsuka K., Wang Y., Sunada E., Yamanaka I., J. Catal., 1998, 175(2), 152—160 |
| [17] | Tang W., Hu Z. P., Wang M. J., Stucky G. D., Metiu H., McFarland E. W., J. Catal., 2010, 273(2), 125—137 |
| [18] | Frind R., Borchardt L., Kockrick E., L Mammitzsch L., Petasch U., Herrmann M., Kaskel S., Catal. Sci. Technol., 2012, 2(1), 139—146 |
| [19] | Fathi M., Bjorgum E., Viig T., Rokstad O. A., Catal. Today,2009, 63(63), 489—497 |
| [20] | Zhu T., Flytzani-Stephanopoulos M., Appl. Catal. A: Gen., 2001, 208(1), 403—417 |
| [21] | Mattos L.V., Oliveira E. R. D., Resende P. D., Noronha F. B., Passos F. B., Catal. Today,2002, 77(3), 245—256 |
| [22] | Takenaka S., Tomikubo Y., Kato E., Otsuka K., Fuel,2004, 83(1), 47—57 |
| [23] | Odier E., Schuurman Y., Mirodatos C., Catal. Today,2007, 127(s1—4), 230—237 |
| [24] | Xu X. L., Han H., Liu J. J., Liu W. M., Li W. L., Wang X., J. Rare Earths,2014, 32(2), 159—169 |
| [25] | Shao J. J., Zhang P., Tang X. F., Zhang B. C., Song W., Xu Y. D., Shen W. J., Chin. J. Catal., 2007, 28(2), 163—169 |
| (邵建军, 张平, 唐幸福, 张保才, 宋巍, 徐奕德, 申文杰. 催化学报, 2007, 28(2), 163—169) | |
| [26] | Alvarez A., Ivanova S., Centeno M. A., Odriozola J. A., Appl. Catal. A: Gen., 2012, 431/432(29), 9—17 |
| [27] | Zhu X., Sun L. Y., Zheng Y. E., Wang H., Wei Y. G., Li K. Z., Int. J. Hydrogen Energy,2014, 39(25), 13381—13388 |
| [28] | Fang P., Lu J., Xiao X., Luo M., J. Rare Earths,2008, 26(2), 250—253 |
| [29] | Wang H., Ye J. L., Liu Y., Li Y. D., Qin Y. N., Catal. Today,2007, 129(3/4), 305—312 |
| [30] | Song Y. B., Yu L., Sun C. Y., Ye F., Fang Y. W., Lin W. M., Chin. J. Catal., 2002, 23(3), 267—270 |
| (宋一兵, 余林 孙长勇, 叶飞, 方奕文, 林维明. 催化学报,, 2002, 23(3), 267—270) | |
| [31] | Chiou J. Y. Z., Peng Y. S., Chen Y. P., Yu S. W., Huang H. H., Chuang C. L., Wang C. B., J. Energy Power Sources,2014, 3(1), 115—122 |
| [32] | Shan W., Luo M., Ying P., Shen W Li C., Appl. Catal. A: Gen., 2003, 246(1), 1—9 |
| [33] | Liotta L. F., di Carlo G., Pantaleo G., Deganello G., Catal. Commun., 2005, 6(5), 329—336 |
| [34] | Xue L., He H., Acta Physico-Chimica Sinica,2007, 23(5), 664—670 |
| (薛莉, 贺泓. 物理化学学报, 2007, 23(5), 664—670) | |
| [35] | Liotta L.F., Carlo G. D., Pantaleo G., Venezia A. M., Deganello G., Appl. Catal. B: Envir., 2006, 66(3), 217—227 |
| [36] | Boskovie G., Smith K. J., Catal. Today,1997, 37(1), 25—32 |
| [37] | Si R., Zhang Y. W., Xiao C. X., Li S. J., Lin B. X., Kou Y., Yan C. H., Phys. Chem. Chem. Phys., 2004, 6(22), 1056—1063 |
| [38] | Dai X. P., Li J., Fan J. T., Wei W. S., Xu J., Ind. Eng. Chem. Res., 2012, 51(34), 11072—11082 |
| [39] | Dai X. P., Yu C. C., Chin. J. Catal., 2011, 32(08), 1411—1417 |
| (代小平, 余长春. 催化学报, 2011, 32(08), 1411—1417) |
| [1] | 何鸿锐, 夏文生, 张庆红, 万惠霖. 羟基氧化铟团簇与二氧化碳和甲烷作用的密度泛函理论研究[J]. 高等学校化学学报, 2022, 43(8): 20220196. |
| [2] | 宋颖颖, 黄琳, 李庆森, 陈立妙. CuO/BiVO4光催化剂的制备及光催化CO2还原性能[J]. 高等学校化学学报, 2022, 43(6): 20220126. |
| [3] | 王明智, 郑燕萍, 翁维正. CeO2负载的PdO与Ce1‒x Pd x O2‒δ 物种的甲烷催化燃烧性能[J]. 高等学校化学学报, 2022, 43(4): 20210816. |
| [4] | 刘华铮, 潘小光, 李华, 万仁忠, 刘希功. Na2CO3催化的叠氮三甲基硅烷对δ-三氟甲基-δ-芳基取代对亚甲基苯醌的1,6-共轭加成: 高效构建含三氟甲基和叠氮取代的二芳基甲烷化合物[J]. 高等学校化学学报, 2021, 42(9): 2772. |
| [5] | 钟声广, 夏文生, 张庆红, 万惠霖. 电中性团簇MCu2Ox(M=Cu2+, Ce4+, Zr4+)上甲烷和二氧化碳直接合成乙酸的理论研究[J]. 高等学校化学学报, 2021, 42(9): 2878. |
| [6] | 胡串串, 庞靖祥, 贺闯闯, 李伟, 孙书涛. Sc(OTf)3催化δ⁃腈基对亚甲基苯醌的1,6⁃共轭烯丙基化: 烯丙基二芳基乙腈类化合物的合成[J]. 高等学校化学学报, 2021, 42(9): 2805. |
| [7] | 李健, 于明明, 孙源, 冯文华, 冯兆池, 吴剑峰. 水溶液pH对甲烷低温氧化制备甲醇的影响[J]. 高等学校化学学报, 2021, 42(3): 776. |
| [8] | 申文杰. 分子围栏催化剂用于甲烷低温氧化制甲醇[J]. 高等学校化学学报, 2020, 41(3): 375. |
| [9] | 吴昊, 王长真, 仇媛, 田亚妮, 赵永祥. Ni-SiO2催化剂空间限域维度对CH4-CO2重整反应金属抗积碳能力的影响[J]. 高等学校化学学报, 2020, 41(11): 2488. |
| [10] | 马静雨, 刘双磊, 张振国, 金俊阳, 贾振华. B(C6F5)3催化合成二吲哚甲烷类化合物的研究[J]. 高等学校化学学报, 2020, 41(10): 2225. |
| [11] | 冉诗雅, 沈海峰, 李晓楠, 王子路, 郭正虹, 方征平. 三氟甲烷磺酸稀土盐对聚丙烯热稳定性的影响及机理[J]. 高等学校化学学报, 2019, 40(6): 1333. |
| [12] | 程文敏, 夏文生, 万惠霖. 氧化镧表面反应性对甲烷和氧分子活化的影响[J]. 高等学校化学学报, 2019, 40(5): 940. |
| [13] | 陈涛,方镭,罗伟,孟跃,薛继龙,夏盛杰,倪哲明. 双金属合金团簇M12Ni(M=Pt, Sn, Cu)催化甲烷干法重整反应的理论研究[J]. 高等学校化学学报, 2019, 40(10): 2135. |
| [14] | 林丹, 程敏, 杜宜奎, 朱起鹤. CF3I在238 nm的光解碎片平动能谱研究:CF3光解碎片的振动态分布和势能曲线交叉穿越几率[J]. 高等学校化学学报, 2018, 39(8): 1713. |
| [15] | 邓贵先, 李孔斋, 程显名, 顾振华, 卢春强, 祝星. 赤泥作为氧载体用于甲烷化学链燃烧:反应与循环性能[J]. 高等学校化学学报, 2018, 39(2): 327. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||