

高等学校化学学报 ›› 2016, Vol. 37 ›› Issue (8): 1421.doi: 10.7503/cjcu20160126
收稿日期:2016-03-03
出版日期:2016-07-14
发布日期:2016-07-14
作者简介:联系人简介: 李夏, 女, 博士, 教授, 博士生导师, 主要从事无机配位化学研究. E-mail:
基金资助:
DONG Gaoyun, LI Rui, FAN Tingting, LI Jiajia, LI Xia*( )
)
Received:2016-03-03
Online:2016-07-14
Published:2016-07-14
Contact:
LI Xia
E-mail:xiali@cnu.edu.cn
Supported by:摘要:
采用水热法合成了5个稀土配合物[Sm2(bdbc)2(phen)4](1)和[Ln(bdbc)(phen)(H2O)][Ln=Eu(2), Gd(3), Tb(4), Dy(5), bdbc=(2-羧基苯氧基)苯-1,2-二羧酸根, phen=1,10-邻菲啰啉]. 配合物1是双核分子, 通过氢键和C—H…π作用进一步构筑成一维超分子结构; 配合物2~5是同构的一维双螺旋结构, 通过氢键和C—H…π作用进一步构筑成三维超分子结构. 配合物1, 2, 4和5呈现了Sm3+, Eu3+, Tb3+和Dy3+离子的特征发射, 分别对应于Sm3+离子的4G5/2→6HJ/2(J=5, 7, 9)、 Eu3+离子的5D0→7FJ(J=1—4)、 Tb3+离子的5D4→7FJ(J=6, 5, 4, 3)和Dy3+离子的4F5/2→6HJ/2(J=15, 13)跃迁. 对配合物4的荧光性质进行了表征, 结果表明, 配合物4可用作荧光探针以检测阳离子和苯甲醛.
中图分类号:
TrendMD:
董高云, 李睿, 樊婷婷, 李佳佳, 李夏. (2-羧基苯氧基)苯-1,2-二羧酸构筑的镧系配合物对苯甲醛和阳离子的荧光传感. 高等学校化学学报, 2016, 37(8): 1421.
DONG Gaoyun,LI Rui,FAN Tingting,LI Jiajia,LI Xia. Luminescence Sensing of Benzaldehyde and Cation of 3-(2-Carboxy-phenoxy)-phthalicate-based Lanthanide Complexes†. Chem. J. Chinese Universities, 2016, 37(8): 1421.
| Compound | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Empirical formula | C78H46N8O14Sm2 | C27H17N2O8Eu | C27H17N2O8Gd | C27H17N2O8Tb | C27H17N2O8Dy |
| Formula weight | 1619.93 | 649.39 | 654.67 | 656.34 | 659.92 |
| Temperature/K | 296(2) | 296(2) | 296(2) | 296(2) | 296(2) |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | P21/n | P21/n | P21/n | P21/n |
| a/nm | 2.79501(11) | 1.17504(5) | 1.17519(4) | 1.1732(2) | 1.17249(4) |
| b/nm | 1.33737(5) | 1.04560(4) | 1.04434(4) | 1.0427(2) | 1.04125(4) |
| c/nm | 2.30816(15) | 2.01377(8) | 2.01048(7) | 2.0085(4) | 2.00662(7) |
| β/(°) | 121.4560(10) | 96.7414(11) | 96.8519(11) | 96.86(3) | 96.8906(10) |
| V/nm3 | 7.3599(6) | 2.45706(17) | 2.44983(15) | 2.4394(9) | 2.43210(15) |
| Z | 4 | 4 | 4 | 4 | 4 |
| Dc/(Mg·m-3) | 1.462 | 1.755 | 1.775 | 1.787 | 1.802 |
| Absorption coefficient/mm-1 | 1.649 | 2.608 | 2.763 | 2.955 | 3.128 |
| F(000) | 3224 | 1280 | 1284 | 1288 | 1292 |
| Crystal size/mm | 0.328×0.069×0.056 | 0.411×0.359×0.189 | 0.339×0.204×0.156 | 0.296×0.230×0.101 | 0.382×0.116×0.091 |
| θ range for data collection /(°) | 2.98—24.51 | 3.19—27.49 | 3.19—27.55 | 3.451—25.098 | 3.20—27.50 |
| Limiting indices | -36≤h≤36, -17≤k≤17, -29≤l≤30 | -14≤h≤13, -12≤k≤12, -24≤l≤24 | -14≤h≤13, -12≤k≤12, -23≤l≤23 | -14≤h≤13, -12≤k≤12, -23≤l≤23 | -13≤h≤13, -12≤k≤12, -23≤l≤23 |
| Reflections collected/unique | 75545/6539 | 31331/4367 | 31544 / 4354 | 32542 / 4341 | 31358 / 4324 |
| Rint | 0.0920 | 0.0256 | 0.0280 | 0.0291 | 0.0326 |
| Completeness(%) | 99.6 | 99.8 | 99.8 | 99.8 | 99.8 |
| Data/restraints/parameters | 8519/0/460 | 4367/1/353 | 4354/1/353 | 4331/1/353 | 4324/1/353 |
| Goodness-of-fit on F2 | 1.037 | 1.064 | 1.037 | 1.082 | 1.076 |
| Final R indices[I>2σ(I)] | R1=0.1082, wR2=0.0991 | R1=0.0225, wR2=0.0471 | R1=0.0240, wR2=0.0434 | R1=0.0229, wR2=0.0441 | R1=0.0288, wR2=0.0459 |
| R indices(all data) | R1=0.0440, wR2=0.0833 | R1=0.0194, wR2 =0.0451 | R1=0.0191, wR2=0.0411 | R1=0.0182, wR2=0.0420 | R1=0.0210, wR2=0.0432 |
| CCDC No. | 1052818 | 1052814 | 1052845 | 1052846 | 1052875 |
Table 1 Crystallographic data of complexes 1—5
| Compound | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Empirical formula | C78H46N8O14Sm2 | C27H17N2O8Eu | C27H17N2O8Gd | C27H17N2O8Tb | C27H17N2O8Dy |
| Formula weight | 1619.93 | 649.39 | 654.67 | 656.34 | 659.92 |
| Temperature/K | 296(2) | 296(2) | 296(2) | 296(2) | 296(2) |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | P21/n | P21/n | P21/n | P21/n |
| a/nm | 2.79501(11) | 1.17504(5) | 1.17519(4) | 1.1732(2) | 1.17249(4) |
| b/nm | 1.33737(5) | 1.04560(4) | 1.04434(4) | 1.0427(2) | 1.04125(4) |
| c/nm | 2.30816(15) | 2.01377(8) | 2.01048(7) | 2.0085(4) | 2.00662(7) |
| β/(°) | 121.4560(10) | 96.7414(11) | 96.8519(11) | 96.86(3) | 96.8906(10) |
| V/nm3 | 7.3599(6) | 2.45706(17) | 2.44983(15) | 2.4394(9) | 2.43210(15) |
| Z | 4 | 4 | 4 | 4 | 4 |
| Dc/(Mg·m-3) | 1.462 | 1.755 | 1.775 | 1.787 | 1.802 |
| Absorption coefficient/mm-1 | 1.649 | 2.608 | 2.763 | 2.955 | 3.128 |
| F(000) | 3224 | 1280 | 1284 | 1288 | 1292 |
| Crystal size/mm | 0.328×0.069×0.056 | 0.411×0.359×0.189 | 0.339×0.204×0.156 | 0.296×0.230×0.101 | 0.382×0.116×0.091 |
| θ range for data collection /(°) | 2.98—24.51 | 3.19—27.49 | 3.19—27.55 | 3.451—25.098 | 3.20—27.50 |
| Limiting indices | -36≤h≤36, -17≤k≤17, -29≤l≤30 | -14≤h≤13, -12≤k≤12, -24≤l≤24 | -14≤h≤13, -12≤k≤12, -23≤l≤23 | -14≤h≤13, -12≤k≤12, -23≤l≤23 | -13≤h≤13, -12≤k≤12, -23≤l≤23 |
| Reflections collected/unique | 75545/6539 | 31331/4367 | 31544 / 4354 | 32542 / 4341 | 31358 / 4324 |
| Rint | 0.0920 | 0.0256 | 0.0280 | 0.0291 | 0.0326 |
| Completeness(%) | 99.6 | 99.8 | 99.8 | 99.8 | 99.8 |
| Data/restraints/parameters | 8519/0/460 | 4367/1/353 | 4354/1/353 | 4331/1/353 | 4324/1/353 |
| Goodness-of-fit on F2 | 1.037 | 1.064 | 1.037 | 1.082 | 1.076 |
| Final R indices[I>2σ(I)] | R1=0.1082, wR2=0.0991 | R1=0.0225, wR2=0.0471 | R1=0.0240, wR2=0.0434 | R1=0.0229, wR2=0.0441 | R1=0.0288, wR2=0.0459 |
| R indices(all data) | R1=0.0440, wR2=0.0833 | R1=0.0194, wR2 =0.0451 | R1=0.0191, wR2=0.0411 | R1=0.0182, wR2=0.0420 | R1=0.0210, wR2=0.0432 |
| CCDC No. | 1052818 | 1052814 | 1052845 | 1052846 | 1052875 |
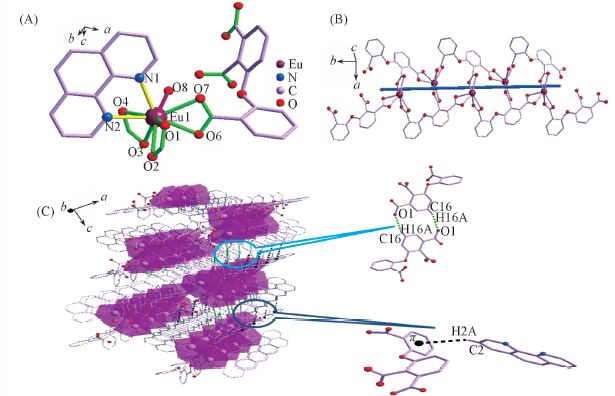
Fig.2 Coordination environment of Eu3+ in complex 2(A), 1D chain structure(B) and 3D supramolecular structure via the hydrogen bonds and C—H…π interactions(C) of complex 2
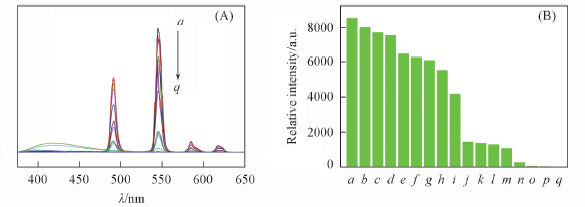
Fig.5 Emission spectra(A) and the 5D4→7F5 transition intensities(B) of complex 4 in the presence of different cations(10-2 mol/L) a. H2O; b. K+; c. Ca2+; d. Li+; e. Mg2+; f. Na+; g. Pb2+; h. Ba2+; i. Zn2+; j. Al3+; k. Cd2+; l. Ni2+; m. Cu2+; n. Ag+; o. Co2+; p. Cr2+; q. Fe3+.
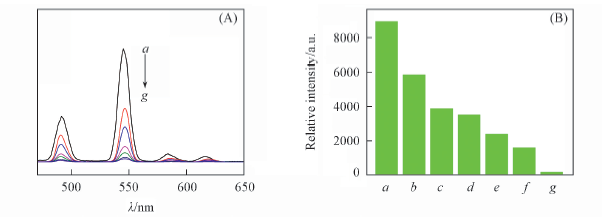
Fig.6 Emission spectra(A) and the 5D4→7F5 transition intensities(B) of complex 4 in the presence of different concentrations of Co2+(λex=350 nm) c(Co2+)/(mol·L-1): a. 0; b. 5×10-6; c. 1×10-5; d. 1×10-4; e. 1×10-3; f. 5×10-3; g. 5×10-2.
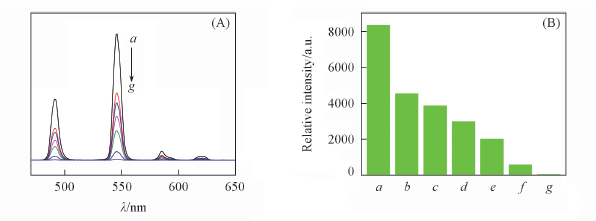
Fig.7 Emission spectra(A) and 5D4→7F5 transition intensities(B) of complex 4 in the presence of different concentrations of Fe3+(λex=350 nm) c(Fe3+)/(mol·L-1): a. 0; b. 5×10-6; c. 1×10-5; d. 1×10-4; e. 1×10-3; f. 5×10-3; g. 5×10-2.
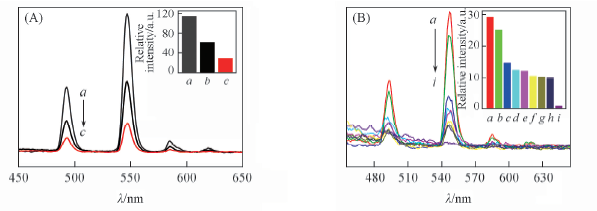
Fig.8 Emission spectra(A, B) and 5D4→7F5 transition intensity(insets) of complex 4 dispersed in different solvents(λex=350 nm) (A) a. EtOAc; b. acetone; c. benzene. (B) a. Benzene; b. dichloromethane; c. methanol; d. formamide; e. dimethylbenzene; f. acetonitrile; g. DMF; h. formaldeltyde; i. benzaldehyde.
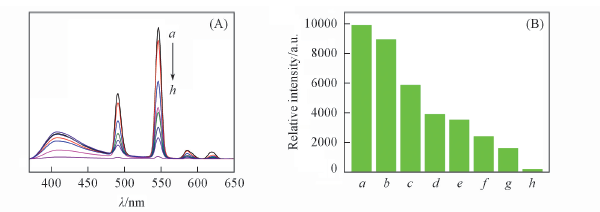
Fig.9 Emission spectra(A) and 5D4→7F5 transition intensity(B) of complex 4 dispersed in EtOAc with different concentrations of benzaldehyde(λex=350 nm) c(Benzaldehyde)/(mol·L-1): a. 10-6; b. 5×10-5; c. 10-4; d. 2×10-4; e. 3×10-4; f. 5×10-4; g. 6×10-4; h. 8×10-4.
| [1] |
Pramanik, S. , Zheng, C. , Zhang, X. , Emge T., J. , Li, J. , J. Am. Chem. Soc., 2011, 133, 4153- 4155
doi: 10.1021/ja106851d URL pmid: 21384862 |
| [2] | Ma L., Q. , Abney, C. , Lin, W. , Chem. Soc. Rev., 2009, 38, 1248- 1256 |
| [3] |
Lee J., Y. , Farha O., K. , Roberts, J. , Scheidt K., A. , Nguyen S., T. , Hupp J., T. , Chem. Soc. Rev., 2009, 38, 1450- 1459
doi: 10.1039/b807080f URL |
| [4] |
Hao J., N. , Yan, B. , Chem. Commun., 2015, 51, 7737- 7740
doi: 10.1007/s12035-015-9439-0 URL pmid: 26392295 |
| [5] |
Zhang Z., J. , Xiang S., C. , Rao X., T. , Zheng, Q. , Fronczek F., R. , Qian G., D. , Chen B., L. , Chem. Commun., 2010, 46, 7205- 7207
doi: 10.1039/c0cc01236j URL pmid: 20737107 |
| [6] | Li J., R. , Kuppler R., J. , Zhou H., C. , Chem. Soc. Rev., 2009, 38, 1477- 1504 |
| [7] |
Wang Y., N. , Zhang, P. , Yu J., H. , Xu J., Q. , Dalton Trans., 2015, 44, 1655- 1663
doi: 10.1186/s12885-015-1887-4 URL pmid: 26541196 |
| [8] | Feng, X. , Wang Y., Y. , Hu Y., C. , Chen S., P. , Zhao W., J. , Yang X., W. , J. Coord. Chem., 2012, 65, 2692- 2704 |
| [9] | Wang, L. , Acta Cryst., 2013, 69, 101- 107 |
| [10] |
Zhang S., Q. , Jiang F., L. , Wu M., Y. , Ma, J. , Bu, Y. , Hong M., C. , Cryst. Growth Des., 2012, 12, 1452- 1463
doi: 10.1021/cg201556b URL |
| [11] |
Wang H., L. , Zhang D., P. , Sun D., F. , Chen Y., T. , Zhang L., F. , Tian L., J. , Jiang J., Z. , Ni Z., H. , Cryst. Growth Des., 2009, 9, 5273- 5282
doi: 10.3959/1536-1098-63.1.27 URL |
| [12] |
Wu, H. , Yang, J. , Su Z., M. , Batten S., R. , Ma J., F. , J. Am. Chem. Soc., 2011, 133, 11406- 11409
doi: 10.1021/ja202303b URL pmid: 21728370 |
| [13] |
Kong C., Y. , J. Inorg. Organomet. Polym. Mater., 2011, 21, 189- 194
doi: 10.1039/C1JM13551A URL |
| [14] |
Celedonio M., A. , Lucia A., M. , Raul G., R. , Eur. J. Inorg. Chem., 2015, 29, 4921- 4934
doi: 10.1002/ejic.201500776 URL |
| [15] |
Marchand, A. , Granzhan, A. , Iida, K. , Tsushima, Y. , Ma, Y. , Nagasawa, K. , Teulade-Fichou, M. , Gabelica, Valerie. , J. Am. Chem. Soc., 2015, 137, 750- 756
doi: 10.1021/ja5099403 URL pmid: 25525863 |
| [16] |
Accorsi, G. , Listorti, A. , Yoosaf, K. , Armaroli, N. , Chem. Soc. Rev., 2009, 38, 1690- 1700
doi: 10.1039/b806408n URL pmid: 19587962 |
| [17] | Bauer C., A. , Timofeeva T., V. , Settersten T., B. , Patterson B., D. , Liu V., H. , Simmons B., A. , Allendorf M., D. , J. Am. Chem. Soc., 2007, 129, 7136- 7144 |
| [18] |
Saleem, M. , Lee K., H. , RSC Adv., 2015, 5, 72150- 72287
doi: 10.1039/C5RA13831K URL |
| [19] |
Novio, F. , Simmchen, J. , Vazquez-Mera, N. , Amorin-Ferre, L. , Ruiz-Molina D., C. , Chem. Rev., 2013, 257, 2839- 2847
doi: 10.1016/j.ccr.2013.04.022 URL |
| [20] |
徐布一, 叶懿, 阮若云, 颜有仪, 廖林川. 高等学校化学学报, 2015, 36( 9), 1667- 1673
doi: 10.7503/cjcu20150228 |
|
Xu B., Y. , Ye, Y. , Ruan R., Y. , Yan Y., Y. , Liao L., C. , Chem. J. Chinese Universities, 2015, 36( 9), 1667- 1673
doi: 10.7503/cjcu20150228 |
|
| [21] |
徐惠, 代艳娜, 单洪岩, 费强, 郇延富, 李光华, 冯国栋. 高等学校化学学报, 2014, 35( 4), 736- 740
doi: 10.7503/cjcu20131096 |
|
Xu, H. , Dai Y., N. , Shan H., Y. , Fei, Q. , Huan Y., F. , Li G., H. , Feng G., D. , Chem. J. Chinese Universities, 2014, 35( 4), 736- 740
doi: 10.7503/cjcu20131096 |
|
| [22] | Zhao, B. , Chen X., Y. , Cheng, P. , Liao D., Z. , Yan S., P. , Jiang Z., H. , J. Am. Chem. Soc., 2004, 126, 15394- 15395 |
| [23] |
Harbuzaru B., V. , Corma, A. , Rey, F. , Atienzar, P. , Ananias, D. , Carlos L., D. , Rocha, J. , Angew. Chem. Int. Ed., 2008, 47, 1080- 1083
doi: 10.1002/anie.200704702 URL pmid: 18183561 |
| [24] |
赵秀巧, 王会, 董丽君, 徐庆红. 高等学校化学学报, 2013, 34( 6), 1318- 1326
doi: 10.7503/cjcu20121156 |
|
Zhao X., Q. , Wang, H. , Dong L., J. , Xun Q., H. , Chem. J. Chinese Universities, 2013, 34( 6), 1318- 1326
doi: 10.7503/cjcu20121156 |
|
| [25] | Zhou, Y. , Chen H., H. , Yan, B. , J. Mater. Chem. A., 2014, 2, 13691- 13697 |
| [26] |
Hao J., N. , Yan, B. , Chem. Commun., 2015, 51, 7737- 7740
doi: 10.1007/s12035-015-9439-0 URL pmid: 26392295 |
| [27] |
Shi B., B. , Zhong Y., H. , Guo L., L. , Dalton Trans., 2015, 44, 4362- 4369
doi: 10.1039/c4dt03326d URL pmid: 25641054 |
| [28] | Sheldrick G., M. , SHELXS 97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, 1997 |
| [29] | Sheldrick G., M. , SHELXL 97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen, 1997 |
| [30] |
Gore A., H. , Gunjal D., B. , Kokate M., R. , Sudarsan, V. , Anbhule P., V. , Patil S., R. , Kolekar G., B. , ACS Appl. Mater. Interfaces, 2012, 4, 5217- 5226
doi: 10.1021/am301136q URL pmid: 22948013 |
| [31] | Zhou, Y. , Chen H., H. , Yan, B. , J. Mater. Chem. A, 2014, 2, 13691- 13697 |
| [32] |
Xiang, Z. , Fang, C. , Leng, S. , Cao, D. , J. Mater. Chem. A, 2014, 2, 7662- 7665
doi: 10.1016/j.ica.2014.09.015 URL |
| [1] | 季双琦, 靳钊, 观文娜, 潘翔宇, 关彤. 双阳离子型离子液体和十八烷基修饰的混合模式硅胶固定相的制备及色谱性能[J]. 高等学校化学学报, 2022, 43(6): 20220008. |
| [2] | 赵永梅, 穆叶舒, 洪琛, 罗稳, 田智勇. 双萘酰亚胺衍生物用于检测水溶液中的苦味酸[J]. 高等学校化学学报, 2022, 43(3): 20210765. |
| [3] | 唐倩, 但飞君, 郭涛, 兰海闯. 喹啉酮-香豆素类Hg2+比色荧光探针的合成及应用[J]. 高等学校化学学报, 2022, 43(2): 20210660. |
| [4] | 周永辉, 李尧, 吴雨轩, 田晶, 徐龙权, 费旭. 一种新型光致发光自愈合水凝胶的合成[J]. 高等学校化学学报, 2022, 43(2): 20210606. |
| [5] | 程媛媛, 郗碧莹. ·OH自由基引发CH3SSC |
| [6] | 王迪, 钟克利, 汤立军, 侯淑华, 吕春欣. 席夫碱共价有机框架的合成及对I ‒ 的识别[J]. 高等学校化学学报, 2022, 43(10): 20220115. |
| [7] | 魏闯宇, 陈艳丽, 姜建壮. 基于乙硫基取代的三层酞菁铕二聚体修饰ITO电极构筑电化学多巴胺和尿酸传感器[J]. 高等学校化学学报, 2022, 43(1): 20210582. |
| [8] | 吴季, 张浩, 骆昱晖, 耿吴越, 兰亚乾. 一种具有荧光性质的阳离子Ga⁃MOF用于Fe3+和硝基化合物识别[J]. 高等学校化学学报, 2022, 43(1): 20210617. |
| [9] | 黄珊, 姚建东, 宁淦, 肖琦, 刘义. 石墨烯量子点荧光探针对碱性磷酸酶活性的高效检测[J]. 高等学校化学学报, 2021, 42(8): 2412. |
| [10] | 卓增庆, 潘锋. 基于软X射线光谱的锂电池材料的电子结构与演变的研究进展[J]. 高等学校化学学报, 2021, 42(8): 2332. |
| [11] | 李安然, 赵冰, 阚伟, 宋天舒, 孔祥东, 卜凡强, 孙立, 殷广明, 王丽艳. 基于菲并咪唑的ON⁃OFF⁃ON双比色荧光探针及细胞成像[J]. 高等学校化学学报, 2021, 42(8): 2403. |
| [12] | 杨新杰, 赖艳琼, 李秋旸, 张艳丽, 王红斌, 庞鹏飞, 杨文荣. 基于环状DNA-银纳米簇荧光探针对微囊藻毒素-LR的传感检测[J]. 高等学校化学学报, 2021, 42(12): 3600. |
| [13] | 谌委菊, 陈诗雅, 薛曹叶, 刘波, 郑晶. 缺氧响应荧光探针的成像及治疗应用[J]. 高等学校化学学报, 2021, 42(11): 3433. |
| [14] | 黄加玲,刘凤娇,王婷婷,刘翠娥,郑凤英,王振红,李顺兴. 氮硫共掺杂碳量子点对胃液pH值的精确检测[J]. 高等学校化学学报, 2020, 41(7): 1513. |
| [15] | 沈扬, 朱方, 沈湾湾, 范倩倩, 李乙文, 程义云. 植物多酚基元辅助递送siRNA的构效关系研究[J]. 高等学校化学学报, 2020, 41(4): 633. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||