

高等学校化学学报 ›› 2016, Vol. 37 ›› Issue (2): 269.doi: 10.7503/cjcu20150465
收稿日期:2015-06-12
出版日期:2016-02-10
发布日期:2016-01-14
作者简介:联系人简介: 童孟良, 男, 教授, 高级工程师, 主要从事金属催化反应研究. E-mail:基金资助:Received:2015-06-12
Online:2016-02-10
Published:2016-01-14
Contact:
TONG Mengliang
E-mail:13973327103@163.com
Supported by:摘要:
以二叔丁基过氧化物(DTBP)为氧化剂, 苯硫酚为硫化试剂, 在无金属参与的条件下, 于120 ℃下采用一步法合成了硫代苄醚. 这种构筑C(sp3)—S键的方法具有高原子经济性和高选择性的优点, 并以较高的收率获得了一系列目标化合物.
中图分类号:
TrendMD:
鄢东, 童孟良. 无金属参与的氧原子邻位C(sp3)—S键的构筑. 高等学校化学学报, 2016, 37(2): 269.
YAN Dong, TONG Mengliang. Metal-free Thiolation of C(sp3)—S Bond Adjacent to an Oxygen Atom†. Chem. J. Chinese Universities, 2016, 37(2): 269.
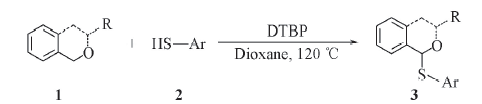
Scheme 1 Thioetherification of Isochromans 3a: R=Me, Ar=Ph; 3b: R=Et, Ar=Ph; 3c: R=i-Pr, Ar=Ph; 3d: R=Ph, Ar=Ph; 3e: R=PhCO, Ar=Ph; 3f: R= Me, Ar=4-Me-Ph; 3g: R=Me, Ar=4-OMe-Ph; 3h: R=Me, Ar=4-Cl-Ph; 3i: R= Me, Ar=4-Me-Ph; 3j: R=i-Pr, Ar=Ph; 3k: R=i-Pr, Ar=4-Cl-Ph; 3l: R=—CH2CH2—, Ar=Ph; 3m: R=—CH2CH2—, Ar=4-Cl-Ph
| Compd. | Appearance | m.p./℃ | HR-MS, m/z([M]+) | IR(KBr), |
|---|---|---|---|---|
| 3a | Yellow oil | — | 230.0758 | 2956, 2854, 1645, 1479, 1387, 1022, 745, 698 |
| 3b | Yellow solid | 67—68 | 244.0913 | 2916, 2848, 1490, 1390, 1014, 810, 723 |
| 3c | Brown solid | 70—72 | 258.1071 | 2935, 2860, 1661, 1507, 1379, 1009, 793 |
| 3d | Pale yellow oil | — | 292.0918 | 3011, 1597, 1495, 1212, 1019, 798, 694 |
| 3e | Brown solid | 87—89 | 320.0866 | 2930, 1634, 1594, 1392, 1226, 1033, 783 |
| 3f | Yellow oil | — | 244.0913 | 3013, 2918, 2843, 1595, 1492, 1238, 1016, 785 |
| 3g | Brown solid | 72—74 | 260.0867 | 2923, 2841, 1593, 1498, 1236, 1012, 831 |
| 3h | Yellow solid | 77—78 | 264.0370 | 2917, 2851, 1478, 1389, 1261, 1101, 1008, 812 |
| 3i | Yellow oil | — | 272.1223 | 2941, 2876, 1497, 1354, 1242, 1104, 1011, 806 |
| 3j | Pale yellow oil | — | 288.1177 | 2923, 2824, 1596, 1369, 1198, 1016, 822, 697 |
| 3k | Yellow oil | — | 292.0678 | 2921, 2837, 1588, 1469, 1382, 1258, 1127, 996, 793 |
| 3l | Yellow solid | 84—86 | 242.0759 | 3012, 2923, 2844, 1596, 1389, 1244, 1012, 803, 698 |
| 3m | Yellow solid | 81—82 | 276.0368 | 3009, 2915, 2832, 1594, 1471, 1254, 1123, 1014, 798 |
Table 1 Appearance, melting points, HR-MS and IR data of compounds 3a—3m
| Compd. | Appearance | m.p./℃ | HR-MS, m/z([M]+) | IR(KBr), |
|---|---|---|---|---|
| 3a | Yellow oil | — | 230.0758 | 2956, 2854, 1645, 1479, 1387, 1022, 745, 698 |
| 3b | Yellow solid | 67—68 | 244.0913 | 2916, 2848, 1490, 1390, 1014, 810, 723 |
| 3c | Brown solid | 70—72 | 258.1071 | 2935, 2860, 1661, 1507, 1379, 1009, 793 |
| 3d | Pale yellow oil | — | 292.0918 | 3011, 1597, 1495, 1212, 1019, 798, 694 |
| 3e | Brown solid | 87—89 | 320.0866 | 2930, 1634, 1594, 1392, 1226, 1033, 783 |
| 3f | Yellow oil | — | 244.0913 | 3013, 2918, 2843, 1595, 1492, 1238, 1016, 785 |
| 3g | Brown solid | 72—74 | 260.0867 | 2923, 2841, 1593, 1498, 1236, 1012, 831 |
| 3h | Yellow solid | 77—78 | 264.0370 | 2917, 2851, 1478, 1389, 1261, 1101, 1008, 812 |
| 3i | Yellow oil | — | 272.1223 | 2941, 2876, 1497, 1354, 1242, 1104, 1011, 806 |
| 3j | Pale yellow oil | — | 288.1177 | 2923, 2824, 1596, 1369, 1198, 1016, 822, 697 |
| 3k | Yellow oil | — | 292.0678 | 2921, 2837, 1588, 1469, 1382, 1258, 1127, 996, 793 |
| 3l | Yellow solid | 84—86 | 242.0759 | 3012, 2923, 2844, 1596, 1389, 1244, 1012, 803, 698 |
| 3m | Yellow solid | 81—82 | 276.0368 | 3009, 2915, 2832, 1594, 1471, 1254, 1123, 1014, 798 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
|---|---|---|
| 3a | 7.17—7.26(m, 10H), 5.67(s, 1H), 3.47(s, 3H) | 139.5, 138.0, 134.3, 133.2, 129.5, 128.1, 127.9, 126.4, 90.2, 56.8 |
| 3b | 7.31(dd, J=4.0, 2.9 Hz, 5H), 7.23(d, J=8.2 Hz, 2H), 7.06(m, 3H), 5.68(s, 1H), 3.42(m, 2H), 1.20(t, J=7.5 Hz, 3H) | 139.4, 138.1, 135.1, 133.2, 129.5, 128.1, 128.1, 127.2, 91.3, 58.2, 21.3 |
| 3c | 7.32—7.30(m, 5H), 7.23(d, J=8.2 Hz, 2H), 7.03(t, J=8.2 Hz, 3H), 5.97(s, 1H), 3.53(m, 1H), 1.29(s, 3H), 1.27(s, 3H) | 138.6, 133.7, 131.4, 129.7, 129.3, 129.0, 128.2, 126.3, 85.0, 73.5, 23.9, 23.8 |
| 3d | 7.34—7.28(m, 5H), 7.24—7.20(m, 5H), 7.11—7.08(t, J=8.2 Hz, 2H), 6.93—6.85(m, 3H), 6.07(s, 1H) | 146.5, 139.0, 134.5, 132.5, 132.3, 130.8, 130.7, 129.4, 129.3, 128.5, 127.6, 125.4, 119.8, 89.2 |
| 3e | 7.97(d, J=6.1 Hz, 2H), 7.47(t, J=7.4 Hz, 1H), 7.40(t, J=7.4 Hz, 2H), 7.35—7.15(m, 10H), 7.09(t, J=7.4 Hz, 1H) | 164.2, 138.9, 133.8, 133.3, 131.4, 131.3, 130.5, 129.8, 129.7, 129.7, 129.6, 129.4, 129.3, 128.8, 128.7, 128.6, 128.4, 125.9, 125.8, 85.3 |
| 3f | 7.48(d, J=7.8 Hz, 2H), 7.40—7.30(m, 1H), 7.26—7.00(m, 5H), 6.45(s, 1H), 4.55—4.52(m, 1H), 3.98(dd, J=11.4, 6.3 Hz, 1H), 3.25—3.05(m, 1H), 2.65—2.63(m, 1H), 2.28(s, 3H) | 137.4, 134.0, 133.9, 132.2, 132.1, 129.8, 128.9, 127.7, 127.2, 126.1, 86.4, 77.4, 77.1, 76.9, 58.2, 27.9, 21.2 |
| 3g | 7.31—7.25(m, 7H), 6.84(d, J=8.2 Hz, 2H), 5.68(s, 1H), 3.82(s, 3H), 3.42(s, 3H) | 141.3, 139.0, 134.3, 133.2, 129.4, 128.2, 127.6, 125.2, 90.2, 58.2, 56.8 |
| 3h | 7.30—7.29(d, J=8.3 Hz, 4H), 7.25—7.22(m, 5H), 5.80(s, 1H), 3.82(s, 3H), 3.38(s, 3H) | 138.3, 136.0, 133.5, 132.8, 132.0, 129.3, 128.2, 127.6, 126.9, 89.2, 55.9 |
| 3i | 7.31—7.22(m, 7H), 7.08(d, J=8.2 Hz, 2H), 5.68(s, 1H), 3.51(m, 2H), 1.27(s, 3H), 1.26(s, 6H) | 139.6, 138.9, 133.3, 131.2, 130.8, 129.1, 128.7, 126.4, 87.9, 78.2, 23.7, 21.2 |
| 3j | 7.32—7.28(m, 6H), 7.24(t, J=7.5 Hz, 1H), 6.98(d, J=8.2 Hz, 2H), 5.68(s, 1H), 3.68(s, 1H), 3.47(m, 1H), 1.24(s, 3H), 1.22(s, 6H) | 142.8, 138.9, 133.3, 131.2, 130.8, 128.7, 126.4, 115.6, 87.9, 75.2, 56.0, 22.7, 21.2 |
| 3k | 7.32—7.28(m, 6H), 7.28—7.21(m, 3H), 5.68(s, 1H), 3.47(m, 1H), 1.24(s, 3H), 1.22(s, 6H) | 138.9, 135.5, 133.6, 132.2, 129.9, 128.9, 128.2, 126.4, 87.9, 75.2, 21.2 |
| 3l | 7.43—7.31(m, 5H), 7.24—7.19(m, 4H), 6.49(s, 1H), 4.55—4.53(m, 2H), 3.20—3.09(m, 1H), 2.72(dd, J=16.5, 2.4 Hz, 1H) | 136.6, 132.9, 132.5, 132.3, 132.2, 128.1, 127.9, 126.9, 126.1, 125.2, 85.1, 57.4, 25.9 |
| 3m | 7.62(d, J=7.8 Hz, 2H), 7.41—7.32(m, 1H), 7.26—7.00(m, 5H), 6.45(s, 1H), 4.55(m, 1H), 3.98(dd, J=11.4, 6.3 Hz, 1H), 3.25—3.05(m, 1H), 2.65(dd, J=16.5, 2.9 Hz, 1H), 2.28(s, 3H) | 138.4, 136.0, 133.5, 132.7, 132.0, 129.3, 128.2, 127.6, 126.9, 126.1, 125.2, 85.1, 57.5, 25.8 |
Table 2 1H NMR, 13C NMR data of compounds 3a—3m
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
|---|---|---|
| 3a | 7.17—7.26(m, 10H), 5.67(s, 1H), 3.47(s, 3H) | 139.5, 138.0, 134.3, 133.2, 129.5, 128.1, 127.9, 126.4, 90.2, 56.8 |
| 3b | 7.31(dd, J=4.0, 2.9 Hz, 5H), 7.23(d, J=8.2 Hz, 2H), 7.06(m, 3H), 5.68(s, 1H), 3.42(m, 2H), 1.20(t, J=7.5 Hz, 3H) | 139.4, 138.1, 135.1, 133.2, 129.5, 128.1, 128.1, 127.2, 91.3, 58.2, 21.3 |
| 3c | 7.32—7.30(m, 5H), 7.23(d, J=8.2 Hz, 2H), 7.03(t, J=8.2 Hz, 3H), 5.97(s, 1H), 3.53(m, 1H), 1.29(s, 3H), 1.27(s, 3H) | 138.6, 133.7, 131.4, 129.7, 129.3, 129.0, 128.2, 126.3, 85.0, 73.5, 23.9, 23.8 |
| 3d | 7.34—7.28(m, 5H), 7.24—7.20(m, 5H), 7.11—7.08(t, J=8.2 Hz, 2H), 6.93—6.85(m, 3H), 6.07(s, 1H) | 146.5, 139.0, 134.5, 132.5, 132.3, 130.8, 130.7, 129.4, 129.3, 128.5, 127.6, 125.4, 119.8, 89.2 |
| 3e | 7.97(d, J=6.1 Hz, 2H), 7.47(t, J=7.4 Hz, 1H), 7.40(t, J=7.4 Hz, 2H), 7.35—7.15(m, 10H), 7.09(t, J=7.4 Hz, 1H) | 164.2, 138.9, 133.8, 133.3, 131.4, 131.3, 130.5, 129.8, 129.7, 129.7, 129.6, 129.4, 129.3, 128.8, 128.7, 128.6, 128.4, 125.9, 125.8, 85.3 |
| 3f | 7.48(d, J=7.8 Hz, 2H), 7.40—7.30(m, 1H), 7.26—7.00(m, 5H), 6.45(s, 1H), 4.55—4.52(m, 1H), 3.98(dd, J=11.4, 6.3 Hz, 1H), 3.25—3.05(m, 1H), 2.65—2.63(m, 1H), 2.28(s, 3H) | 137.4, 134.0, 133.9, 132.2, 132.1, 129.8, 128.9, 127.7, 127.2, 126.1, 86.4, 77.4, 77.1, 76.9, 58.2, 27.9, 21.2 |
| 3g | 7.31—7.25(m, 7H), 6.84(d, J=8.2 Hz, 2H), 5.68(s, 1H), 3.82(s, 3H), 3.42(s, 3H) | 141.3, 139.0, 134.3, 133.2, 129.4, 128.2, 127.6, 125.2, 90.2, 58.2, 56.8 |
| 3h | 7.30—7.29(d, J=8.3 Hz, 4H), 7.25—7.22(m, 5H), 5.80(s, 1H), 3.82(s, 3H), 3.38(s, 3H) | 138.3, 136.0, 133.5, 132.8, 132.0, 129.3, 128.2, 127.6, 126.9, 89.2, 55.9 |
| 3i | 7.31—7.22(m, 7H), 7.08(d, J=8.2 Hz, 2H), 5.68(s, 1H), 3.51(m, 2H), 1.27(s, 3H), 1.26(s, 6H) | 139.6, 138.9, 133.3, 131.2, 130.8, 129.1, 128.7, 126.4, 87.9, 78.2, 23.7, 21.2 |
| 3j | 7.32—7.28(m, 6H), 7.24(t, J=7.5 Hz, 1H), 6.98(d, J=8.2 Hz, 2H), 5.68(s, 1H), 3.68(s, 1H), 3.47(m, 1H), 1.24(s, 3H), 1.22(s, 6H) | 142.8, 138.9, 133.3, 131.2, 130.8, 128.7, 126.4, 115.6, 87.9, 75.2, 56.0, 22.7, 21.2 |
| 3k | 7.32—7.28(m, 6H), 7.28—7.21(m, 3H), 5.68(s, 1H), 3.47(m, 1H), 1.24(s, 3H), 1.22(s, 6H) | 138.9, 135.5, 133.6, 132.2, 129.9, 128.9, 128.2, 126.4, 87.9, 75.2, 21.2 |
| 3l | 7.43—7.31(m, 5H), 7.24—7.19(m, 4H), 6.49(s, 1H), 4.55—4.53(m, 2H), 3.20—3.09(m, 1H), 2.72(dd, J=16.5, 2.4 Hz, 1H) | 136.6, 132.9, 132.5, 132.3, 132.2, 128.1, 127.9, 126.9, 126.1, 125.2, 85.1, 57.4, 25.9 |
| 3m | 7.62(d, J=7.8 Hz, 2H), 7.41—7.32(m, 1H), 7.26—7.00(m, 5H), 6.45(s, 1H), 4.55(m, 1H), 3.98(dd, J=11.4, 6.3 Hz, 1H), 3.25—3.05(m, 1H), 2.65(dd, J=16.5, 2.9 Hz, 1H), 2.28(s, 3H) | 138.4, 136.0, 133.5, 132.7, 132.0, 129.3, 128.2, 127.6, 126.9, 126.1, 125.2, 85.1, 57.5, 25.8 |
| Entry | Oxidant | n(Oxidant)/mmol | T/℃ | Solvent | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | DTBP | 4.0 | 120 | Dioxane | 39 |
| 2 | K2S2O8 | 4.0 | 120 | Dioxane | |
| 3 | TBHP | 4.0 | 120 | Dioxane | 20 |
| 4 | DDQ | 4.0 | 120 | Dioxane | 12 |
| 5 | BPO | 4.0 | 120 | Dioxane | Trace |
| 6 | DTBP | 3.0 | 120 | Dioxane | 44 |
| 7 | DTBP | 2.0 | 120 | Dioxane | 53 |
| 8 | DTBP | 1.5 | 120 | Dioxane | 80 |
| 9 | DTBP | 1.0 | 120 | Dioxane | 32 |
| 10 | DTBP | 1.5 | 100 | Dioxane | 58 |
| 11 | DTBP | 1.5 | 140 | Dioxane | 67 |
| 12 | DTBP | 1.5 | 120 | Benzene | 69 |
| 13 | DTBP | 1.5 | 120 | PhCl | 56 |
| 14 | DTBP | 1.5 | 120 | Toluene | 13 |
Table 3 Optimization of the reactions between thiophenol and benzyl ethera
| Entry | Oxidant | n(Oxidant)/mmol | T/℃ | Solvent | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | DTBP | 4.0 | 120 | Dioxane | 39 |
| 2 | K2S2O8 | 4.0 | 120 | Dioxane | |
| 3 | TBHP | 4.0 | 120 | Dioxane | 20 |
| 4 | DDQ | 4.0 | 120 | Dioxane | 12 |
| 5 | BPO | 4.0 | 120 | Dioxane | Trace |
| 6 | DTBP | 3.0 | 120 | Dioxane | 44 |
| 7 | DTBP | 2.0 | 120 | Dioxane | 53 |
| 8 | DTBP | 1.5 | 120 | Dioxane | 80 |
| 9 | DTBP | 1.0 | 120 | Dioxane | 32 |
| 10 | DTBP | 1.5 | 100 | Dioxane | 58 |
| 11 | DTBP | 1.5 | 140 | Dioxane | 67 |
| 12 | DTBP | 1.5 | 120 | Benzene | 69 |
| 13 | DTBP | 1.5 | 120 | PhCl | 56 |
| 14 | DTBP | 1.5 | 120 | Toluene | 13 |
| Entry | Compd. | Yield(%)b | Entry | Compd. | Yield(%)b |
|---|---|---|---|---|---|
| 1 |  | 79 | 7 |  | 82 |
| 2 |  | 78 | 8 |  | 74 |
| 3 |  | 82 | 9 |  | 86 |
| 4 |  | 79 | 10 |  | 81 |
| 5 |  | 77 | 11 |  | 76 |
| 6 |  | 80 | 12 |  | 76 |
Table 4 Scope of the reactiona
| Entry | Compd. | Yield(%)b | Entry | Compd. | Yield(%)b |
|---|---|---|---|---|---|
| 1 |  | 79 | 7 |  | 82 |
| 2 |  | 78 | 8 |  | 74 |
| 3 |  | 82 | 9 |  | 86 |
| 4 |  | 79 | 10 |  | 81 |
| 5 |  | 77 | 11 |  | 76 |
| 6 |  | 80 | 12 |  | 76 |
| [1] | Li C. J., Acc. Chem. Res., 2009, 42(2), 335—344 |
| [2] | Yi W. G., Yan D., Wu C., Lan L. X., Chem. J. Chinese Universities, 2014, 35(12), 2563—2566 |
| (易卫国, 鄢东, 吴超, 兰立新. 高等学校化学学报, 2014, 35(12), 2563—2566) | |
| [3] | Zhang Y., Feng B. N., Chin. J. Org. Chem., 2014, 34(12), 2406—2411 |
| (张艳, 冯柏年. 有机化学, 2014, 34(12), 2406—2411) | |
| [4] | Kumar R. A., Saidulu G., Prasad K. R., Kumar G. S., Sridhar B., Reddy K. R., Adv. Synth. Catal., 2012, 354(16), 2985—2991 |
| [5] | Chu L., Qing F. L., Chem. Commun., 2010, 46, 6285—6287 |
| [6] | Wu C., Huang W. Y., He W. M., Xiang J. N., Chem. Lett., 2013, 42, 1233—1234 |
| [7] | Shu X. Z., Xia X. F., Yang Y. F., Ji K. G., Liu X. Y., Liang Y. M., J. Org. Chem., 2009, 74(19), 7464—7469 |
| [8] | He T., Yu L., Zhang L., Wang L., Wang M., Org. Lett., 2011, 13(19), 5016—5019 |
| [9] | Chen L., Shi E., Liu Z., Chen S., Wei W., Li H., Xu K., Wan X., Chem. Eur. J., 2011, 17(15), 4085—4089 |
| [10] | Li X., Wang H. Y., Shi Z. J., New J. Chem., 2013, 37, 1704—1706 |
| [11] | Chen D., Pan F. Y., Gao J. R., Yang J. G., Synlett., 2013, 24(16), 2085—2088 |
| [12] | Xiang S. K., Zhang B., Zhang L. H., Cui Y. X., Jiao N., Sci. China Chem., 2012, 55(1), 50—54 |
| [13] | Correia C. A., Li C. J., Heterocycles, 2010, 82(1), 555—562 |
| [14] | Zhang Y. H., Li C. J., J. Am. Chem. Soc., 2006, 128(13), 4242—4243 |
| [15] | Kang X., Yan R. L., Yu G. Q., Pang X. B., Liu X. X., Li X. N., Xiang L. K., Huang G. S., J. Org. Chem., 2014, 79(21), 10605—10610 |
| [16] | Varun B. V., Prabhu K. R., J. Org. Chem., 2014, 79(20), 9655—9668 |
| [17] | Corbet J. P., Mignani G., Chem. Rev., 2006, 106(7), 2651—2710 |
| [18] | Frei R., Waser J., J. Am. Chem. Soc., 2013, 135(26), 9620—9623 |
| [19] | Saravanan P., Anbarasan P., Org. Lett., 2014, 16(3), 848—851 |
| [20] | Zhao J., Xuan L. N., Zhao H. C., Cheng J., Fu X. Y., Li S., Jing F., Liu Y. M., Chen B. Q., Chem. Res. Chinese Universities, 2014, 30(5), 764—769 |
| [21] | Guo S. R., Yuan Y. Q., Xiang J. N., Org. Lett., 2013, 15(18), 4654—4657 |
| [1] | 林高鑫, 王家成. 单原子掺杂二硫化钼析氢催化的进展和展望[J]. 高等学校化学学报, 2022, 43(9): 20220321. |
| [2] | 张之材, 王玉阁, 谷倩倩, 吕永鹏, 肖建数, 尹园, 孙洪国, 郑雅芳, 孙昭艳. 异戊橡胶中填料的絮凝及其对性能的影响[J]. 高等学校化学学报, 2022, 43(8): 20220155. |
| [3] | 王广琦, 毕艺洋, 王嘉博, 石洪飞, 刘群, 张钰. 非贵金属三元复合Ni(PO3)2-Ni2P/CdS NPs异质结的构建及可见光高效催化产氢性能[J]. 高等学校化学学报, 2022, 43(6): 20220050. |
| [4] | 尹肖菊, 孙逊, 赵程浩, 姜波, 赵晨阳, 张乃庆. 单原子催化剂在锂硫电池中的研究进展[J]. 高等学校化学学报, 2022, 43(5): 20220076. |
| [5] | 孟祥龙, 杨歌, 郭海玲, 刘晨光, 柴永明, 王纯正, 郭永梅. 纳米分子筛的合成及硫化氢吸附性能[J]. 高等学校化学学报, 2022, 43(3): 20210687. |
| [6] | 张诗昱, 何润合, 李永兵, 魏士俊, 张兴祥. 辐照交联制备低分子量聚丙烯腈纤维锂硫电池正极材料及其储硫机理[J]. 高等学校化学学报, 2022, 43(3): 20210632. |
| [7] | 陈铭苏, 张会茹, 张琪, 刘家琴, 吴玉程. 锂硫电池中钴磷共掺杂MoS2催化性能的第一性原理研究[J]. 高等学校化学学报, 2021, 42(8): 2540. |
| [8] | 杨晓梅, 吴强, 郭茹, 叶凯波, 薛屏, 王晓中, 赖小勇 . 超薄骨架有序介孔CdS/NiS的制备及光催化产氢性能[J]. 高等学校化学学报, 2021, 42(5): 1581. |
| [9] | 张楠, 韩阔, 李悦, 王春茹, 赵凤, 韩冬雪, 牛利. 尖晶石型过渡金属硫化物CuCo2S4与MoS2复合材料的制备及电催化析氢性能[J]. 高等学校化学学报, 2021, 42(4): 1307. |
| [10] | 黄岩, 张树鑫, 努丽燕娜, 王保峰, 杨军, 王久林. 用于镁硫电池的原位生长NiS的泡沫镍正极集流体[J]. 高等学校化学学报, 2021, 42(3): 794. |
| [11] | 黄东雪, 章颖, 曾婷, 张媛媛, 万其进, 杨年俊. 基于过渡金属硫化物/还原氧化石墨烯复合物的高性能超级电容器[J]. 高等学校化学学报, 2021, 42(2): 643. |
| [12] | 陈晓煜, 于然波. 纳米二硫化钼的掺杂及催化电解水产氢的研究进展[J]. 高等学校化学学报, 2021, 42(2): 475. |
| [13] | 余强敏, 张致远, 罗雨婷, 李洋, 成会明, 刘碧录. 金属性二维过渡金属硫化物的溶剂热合成及电催化析氢性能[J]. 高等学校化学学报, 2021, 42(2): 654. |
| [14] | 崔金萍, 陈温贤, 郁非繁, 曹诗雨, 吕维扬, 姚玉元. 碳掺杂六方氮化硼/二硫化钼吸附还原六价铬和助催化降解有机污染物[J]. 高等学校化学学报, 2021, 42(10): 3125. |
| [15] | 周墨林, 蒋欣, 易婷, 杨向光, 张一波. 硫化物固态电解质Li10GeP2S12与锂金属间界面稳定性的改善研究[J]. 高等学校化学学报, 2020, 41(8): 1810. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
