

高等学校化学学报 ›› 2014, Vol. 35 ›› Issue (8): 1720.doi: 10.7503/cjcu20140327
收稿日期:2014-04-08
出版日期:2014-08-10
发布日期:2019-08-01
作者简介:联系人简介: 刘楠楠, 女, 博士, 讲师, 主要从事理论化学研究. E-mail: 基金资助:
LIU Nannan1,*( ), DING Yihong2,*(
), DING Yihong2,*( )
)
Received:2014-04-08
Online:2014-08-10
Published:2019-08-01
Contact:
LIU Nannan,DING Yihong
E-mail:liunann.yl@gmail.com;yhdd@jlu.edu.cn
Supported by:摘要:
对金属多重键配合物Cp2M2(μ-B4N4H8)(M=V, Cr, Mn, Fe)的结构和成键进行了理论研究, 并与Cp2M2(μ-C8H8)进行对比. 计算结果表明, 在Cp2M2(μ-B4N4H8)基态构型中, B4N4H8配体均以硼为桥原子, 金属原子的配位数均为5. 其中, Cp2M2(μ-B4N4H8)(M=V, Cr, Mn)基态的结构和成键都与Cp2M2(μ-C8H8)非常接近; 而Cp2Fe2(μ-B4N4H8)基态结构与Fe为4配位的Cp2Fe2(μ-C8H8)有所不同. Cp2M2(μ-B4N4H8)(M=V, Cr, Mn, Fe)基态结构分别为含V-V三重键的三态、 含Cr-Cr三重键的单态、 含Mn-Mn双键的三态及含Fe-Fe单键的单态.
中图分类号:
TrendMD:
刘楠楠, 丁益宏. Cp2M2(μ-B4N4H8)(M=V,Cr,Mn,Fe)金属多重键成键性质的理论研究. 高等学校化学学报, 2014, 35(8): 1720.
LIU Nannan, DING Yihong. Theoretical Studies on the Bonding Properties of Metal-metal Multiple Bond of Cp2M2(μ-B4N4H8)(M=V,Cr,Mn,Fe)†. Chem. J. Chinese Universities, 2014, 35(8): 1720.
| Complex | da(M-M)/ nm | ΔE/ (kJ·mol-1) | db(M-M)/ nm | ΔE/ (kJ·mol-1) | dc(M-M)/ nm | ΔE/ (kJ·mol-1) | dd(M-M)/ nm |
|---|---|---|---|---|---|---|---|
| Cp2V2(μ-B4N4H8)-Iso1-singlet | 0.228 | 0 | 0.218 | 0 | 0.227 | 0 | |
| Cp2V2(μ-B4N4H8)-Iso1-triplet | 0.245 | -13.6 | 0.240 | -73.0 | 0.244 | -10.8 | |
| Cp2V2(μ-B4N4H8)-Iso2-singlet | 0.240 | 106.0 | 0.232 | 99.6 | 0.239 | 106.8 | |
| Cp2V2(μ-B4N4H8)-Iso2-triplet | 0.260 | 113.6 | 0.259 | 37.9 | 0.260 | 114.6 | |
| Cp2V2(μ-C8H8)-singlet[ | 0.232 | 0 | 0.225 | 0 | 0.231 | 0 | |
| Cp2V2(μ-C8H8)-triplet[ | 0.248 | -6.3 | 0.245 | -67.4 | 0.248 | -6.3 | 0.244[ |
| Cp2Cr2(μ-B4N4H8)-Iso1-singlet | 0.229 | 0 | 0.224 | 0 | 0.228 | 0 | |
| Cp2Cr2(μ-B4N4H8)-Iso1-triplet | 0.245 | 22.3 | 0.259 | -36.2 | 0.245 | 21.3 | |
| Cp2Cr2(μ-B4N4H8)-Iso2-singlet | 0.247 | 96.3 | 0.252 | 53.4 | 0.248 | 94.6 | |
| Cp2Cr2(μ-B4N4H8)-Iso2-triplet | 0.260 | 115.1 | 0.285 | 41.5 | 0.260 | 112.9 | |
| Cp2Cr2(μ-C8H8)-singlet[ | 0.235 | 0 | 0.235 | 0 | 0.234 | 0 | 0.239[ |
| Cp2Cr2(μ-C8H8)-triplet[ | 0.248 | 19.7 | 0.256 | -33.8 | 0.248 | 19.7 | |
| Cp2Mn2(μ-B4N4H8)-Iso1-singlet | 0.266 | 0 | 0.258 | 0 | 0.262 | 0 | |
| Cp2Mn2(μ-B4N4H8)-Iso1-triplet | 0.262 | -42.2 | 0.288 | -38.4 | 0.262 | -28.2 | |
| Cp2Mn2(μ-B4N4H8)-Iso2-singlet | 0.283 | 64.3 | 0.289 | 40.9 | 0.284 | 71.1 | |
| Cp2Mn2(μ-C8H8)-singlet | 0.266 | 0 | 0.268 | 0 | 0.267 | 0 | |
| Cp2Mn2(μ-C8H8)-triplet | 0.270 | -12.1 | 0.265 | 5.1 | 0.267 | -6.7 | |
| Cp2Fe2(μ-B4N4H8)-Iso1-singlet | 0.293 | 0 | 0.295 | 0 | 0.295 | 0 | |
| Cp2Fe2(μ-B4N4H8)-Iso1-triplet | 0.295 | 88.7 | 0.293 | 51.1 | 0.297 | 82.1 | |
| Cp2Fe2(μ-C8H8)-singlet | 0.294 | 0 | 0.298 | 0 | 0.295 | 0 | |
| Cp2Fe2(μ-C8H8)-triplet | 0.343 | 79.5 | 0.348 | 7.8 | 0.346 | 71.7 |
Table 1 Bond lengths of M-M and energies of Cp2M2(μ-B4N4H8) and Cp2M2(μ-C8H8) systems
| Complex | da(M-M)/ nm | ΔE/ (kJ·mol-1) | db(M-M)/ nm | ΔE/ (kJ·mol-1) | dc(M-M)/ nm | ΔE/ (kJ·mol-1) | dd(M-M)/ nm |
|---|---|---|---|---|---|---|---|
| Cp2V2(μ-B4N4H8)-Iso1-singlet | 0.228 | 0 | 0.218 | 0 | 0.227 | 0 | |
| Cp2V2(μ-B4N4H8)-Iso1-triplet | 0.245 | -13.6 | 0.240 | -73.0 | 0.244 | -10.8 | |
| Cp2V2(μ-B4N4H8)-Iso2-singlet | 0.240 | 106.0 | 0.232 | 99.6 | 0.239 | 106.8 | |
| Cp2V2(μ-B4N4H8)-Iso2-triplet | 0.260 | 113.6 | 0.259 | 37.9 | 0.260 | 114.6 | |
| Cp2V2(μ-C8H8)-singlet[ | 0.232 | 0 | 0.225 | 0 | 0.231 | 0 | |
| Cp2V2(μ-C8H8)-triplet[ | 0.248 | -6.3 | 0.245 | -67.4 | 0.248 | -6.3 | 0.244[ |
| Cp2Cr2(μ-B4N4H8)-Iso1-singlet | 0.229 | 0 | 0.224 | 0 | 0.228 | 0 | |
| Cp2Cr2(μ-B4N4H8)-Iso1-triplet | 0.245 | 22.3 | 0.259 | -36.2 | 0.245 | 21.3 | |
| Cp2Cr2(μ-B4N4H8)-Iso2-singlet | 0.247 | 96.3 | 0.252 | 53.4 | 0.248 | 94.6 | |
| Cp2Cr2(μ-B4N4H8)-Iso2-triplet | 0.260 | 115.1 | 0.285 | 41.5 | 0.260 | 112.9 | |
| Cp2Cr2(μ-C8H8)-singlet[ | 0.235 | 0 | 0.235 | 0 | 0.234 | 0 | 0.239[ |
| Cp2Cr2(μ-C8H8)-triplet[ | 0.248 | 19.7 | 0.256 | -33.8 | 0.248 | 19.7 | |
| Cp2Mn2(μ-B4N4H8)-Iso1-singlet | 0.266 | 0 | 0.258 | 0 | 0.262 | 0 | |
| Cp2Mn2(μ-B4N4H8)-Iso1-triplet | 0.262 | -42.2 | 0.288 | -38.4 | 0.262 | -28.2 | |
| Cp2Mn2(μ-B4N4H8)-Iso2-singlet | 0.283 | 64.3 | 0.289 | 40.9 | 0.284 | 71.1 | |
| Cp2Mn2(μ-C8H8)-singlet | 0.266 | 0 | 0.268 | 0 | 0.267 | 0 | |
| Cp2Mn2(μ-C8H8)-triplet | 0.270 | -12.1 | 0.265 | 5.1 | 0.267 | -6.7 | |
| Cp2Fe2(μ-B4N4H8)-Iso1-singlet | 0.293 | 0 | 0.295 | 0 | 0.295 | 0 | |
| Cp2Fe2(μ-B4N4H8)-Iso1-triplet | 0.295 | 88.7 | 0.293 | 51.1 | 0.297 | 82.1 | |
| Cp2Fe2(μ-C8H8)-singlet | 0.294 | 0 | 0.298 | 0 | 0.295 | 0 | |
| Cp2Fe2(μ-C8H8)-triplet | 0.343 | 79.5 | 0.348 | 7.8 | 0.346 | 71.7 |
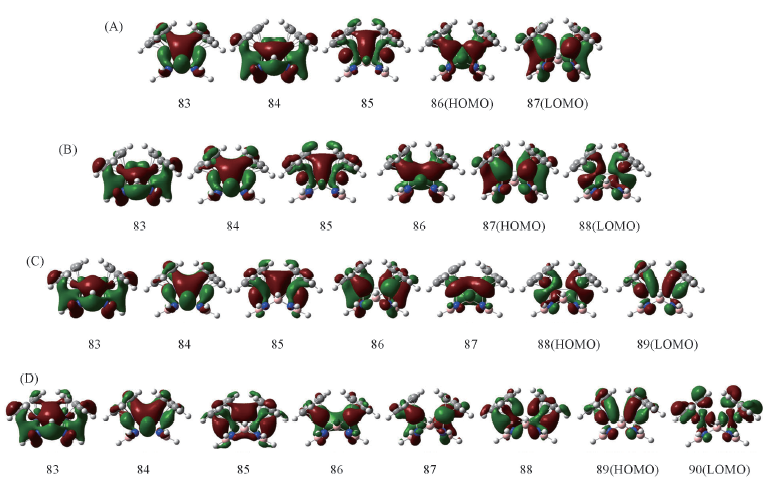
Fig.2 Molecular orbitals of the singlet state of Cp2V2(μ-B4N4H8)-Iso1(A), Cp2Cr2(μ-B4N4H8)-Iso1(B), Cp2Mn2(μ-B4N4H8)-Iso1(C) and Cp2Fe2(μ-B4N4H8)-Iso1(D) Nos. 83—89 represent molecular orbitals related to the metal-metal bonds.
| [1] | Paetzold P., Phosphorus, Sulfur,Silicon, 1994, 93/94, 39—50 |
| [2] | Zhao J. X., Zhang G. L., Dai B. Q., Chem. J. Chinese Universities, 2005, 26(2), 304—307 |
| (赵景祥, 张桂玲, 戴柏青. 高等学校化学学报, 2005, 26(2)), 304—307) | |
| [3] | Zhou Z. J., Li X. P., Huang X. R., Wu Z. J., Li Z. R., Chem. J. Chinese Universities, 2013, 34(9), 2152—2157 |
| (周中军, 李晓平, 黄旭日, 武志坚, 李志儒. 高等学校化学学报, 2013, 34(9), 2152—2157) | |
| [4] | Yue S. M., Tan K., Zhang M., Lan Y. Q., Su Z. M., Chem. J. Chinese Universities, 2003, 24(12), 2231—2234 |
| (岳淑美, 谭克, 张珉, 兰亚乾, 苏忠民. 高等学校化学学报, 2003, 24(12), 2231—2234) | |
| [5] | Engelberts J. J., Havenith R. W. A., van Lenthe J. H., Jenneskens L. W., Fowler P. W., Inorg. Chem., 2005, 44, 5266—5272 |
| [6] | Islas R., Chamorro E., Robles J., Heine T., Santos J. C., Merino G., Struct. Chem., 2007, 18, 833—839 |
| [7] | Mallajosyula S. S., Paridaa P., Pati S. K., J. Mater. Chem ., 2009, 19, 1761—1766 |
| [8] | Yang Z., Liu S., Liu X., Yang Y., Li X., Xiong S., Xu B., J. Phys.: Condens. Matter., 2012, 24(44), 445501-1—M445501-9 |
| [9] | Fowler P. W., Bean D. E., Seed M., J. Phys. Chem. A, 2010, 114, 10742—10749 |
| [10] | Wang H. M., Yang C. L., Wan B. S., Han K. L., J. Theor. Comput. Chem., 2006, 5, 461—473 |
| [11] | Richter U., Heck J., Reinhold J., Inorg. Chem., 2000, 39(4), 658—665 |
| [12] | Sha J. Q., Li X., Peng J., Zong X. M., Zhang Y. H., Yan H., Chem. Res. Chinese Universities, 2011, 27(2), 166—169 |
| [13] | Zhang H., Wang L. P., Shi S. H., Huang L. L., Wang L., Chem. Res. Chinese Universities, 2012, 28(4), 563—566 |
| [14] | Tan Y., Huang X., Xu X., Xu Z. G., Chem. J. Chinese Universities, 2012, 33(6), 1278—1284 |
| (谭莹, 黄晓, 许旋, 徐志广. 高等学校化学学报, 2012, 33(6), 1278—1284) | |
| [15] | Shi S. G., Feng J. K., Tian W. Q., Liu Z. Z., Li W. Q., Cui Y. H., Chem. J. Chinese Universities, 2013, 34(2), 455—461 |
| (时圣刚, 封继康, 田维全, 刘子忠, 李伟奇, 崔艳红. 高等学校化学学报, 2013, 34(2), 455—461) | |
| [16] | Zhai X., Li G., Li Q., Xie Y., King R. B., Schaefer H. F., J. Phys. Chem. A, 2011, 115(14), 3133—3143 |
| [17] | Perdew J. P., Burke K., Ernzerhof M., Phys. Rev. Lett., 1996, 77(18), 3865—3868 |
| [18] | Perdew J. P., Burke K., Ernzerhof M., Phys. Rev. Lett., 1997, 78(7), 1396 |
| [19] | Schuchardt K. L., Didier B. T., Elsethagen T., Sun L., Gurumoorthi V., Chase J., Li J., Windus T. L., J. Chem. Inf. Model., 2007, 47, 1045—1052 |
| [20] | Becke A. D., Phys. Rev. A, 1988, 38(6), 3098—3100 |
| [21] | Becke A. D., J. Chem. Phys., 1993, 98(2), 1372—1377 |
| [22] | Lee C., Yang W., Parr R. G., Phys. Rev. B, 1988, 37(2), 785—789 |
| [23] | Perdew J. P., Phys. Rev. B, 1986, 33(12), 8822—8824 |
| [24] | Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., Nakatsuji H., Caricato M., Li X., Hratchian H.P., Izmaylov A.F., Bloino J., Zheng G., Sonnenberg J.L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam N. J., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R. E., Stratmann O., Yazyev A. J., Austin R., Cammi C., Pomelli J. W., Ochterski R., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision A.1, Gaussian Inc., Wallingford CT, 2009 |
| [25] | Reiher M., Salomon O., Hess B. A., Theor. Chem. Acc., 2001, 107(1), 48—55 |
| [26] | Liu N. N., Ding Y. H., Liu S. P., Chem. J. Chinese Universities, 2014, 35(2), 319—324 |
| (刘楠楠, 丁益宏, 刘树萍. 高等学校化学学报, 2014, 35(2), 319—324) | |
| [27] | Elschenbroich C., Heck J., Massa W., Nun E., Schmidt R., J. Am. Chem. Soc., 1983, 105(9), 2905—2907 |
| [28] | Elschenbroich C., Heck J., Massa W., Schmidt R., Angew. Chem. Int. Ed., 1983, 22(4), 330—331 |
| [29] | Heck J., Rist G., J. Organomet. Chem., 1988, 342, 45—69 |
| [1] | 张咪, 田亚锋, 高克利, 侯华, 王宝山. 三氟甲基磺酰氟绝缘介质理化特性的分子动力学模拟[J]. 高等学校化学学报, 2022, 43(11): 20220424. |
| [2] | 刘洋, 李旺昌, 张竹霞, 王芳, 杨文静, 郭臻, 崔鹏. Sc3C2@C80与[12]CPP纳米环之间非共价相互作用的理论研究[J]. 高等学校化学学报, 2022, 43(11): 20220457. |
| [3] | 王思佳 侯璐 李成龙 李文翠 陆安慧. 空腔型纳米炭的制备与应用[J]. 高等学校化学学报, 0, (): 20220637. |
| [4] | 武晴滢, 祝震予, 吴剑鸣, 徐昕. 泛Kennard-Stone算法的数据集代表性度量与分块采样策略[J]. 高等学校化学学报, 2022, 43(10): 20220397. |
| [5] | 王园月, 安梭梭, 郑旭明, 赵彦英. 5-巯基-1, 3, 4-噻二唑-2-硫酮微溶剂团簇的光谱和理论计算研究[J]. 高等学校化学学报, 2022, 43(10): 20220354. |
| [6] | 张伶育, 张继龙, 曲泽星. RDX分子内振动能量重分配的动力学研究[J]. 高等学校化学学报, 2022, 43(10): 20220393. |
| [7] | 沈琦 陈海瑶 高登辉 赵 熹 那日松 刘佳 黄旭日. 天然产物法卡林二醇与人类 GABAA 受体的相互作用机制研究[J]. 高等学校化学学报, 0, (): 0. |
| [8] | 陈少臣 程敏 王诗慧 吴金奎 罗磊 薛小雨 吉旭 张长春 周利. 预测金属有机骨架的甲烷和氢气输送能力的迁移学习建模[J]. 高等学校化学学报, 0, (): 20220459. |
| [9] | 彭辛哲, 葛娇阳, 王访丽, 余国静, 冉雪芹, 周栋, 杨磊, 解令海. 一种基于苯并噻吩平面格的张力与重组能的理论研究[J]. 高等学校化学学报, 0, (): 20220313. |
| [10] | 郭程, 张威, 唐云. 有序介孔材料: 历史、 现状与发展趋势[J]. 高等学校化学学报, 2022, 43(8): 20220167. |
| [11] | 汤乔伟 蔡小青 李江 诸颖 王丽华 田阳 樊春海 胡钧. 同步辐射X射线成像技术在脑成像研究中的应用[J]. 高等学校化学学报, 0, (): 20220379. |
| [12] | 杨丹, 刘旭, 戴翼虎, 祝艳, 杨艳辉. 金团簇电催化二氧化碳还原反应的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220198. |
| [13] | 戴卫, 侯华, 王宝山. 七氟异丁腈负离子结构与反应活性的理论研究[J]. 高等学校化学学报, 2022, 43(6): 20220044. |
| [14] | 施耐克, 张娅, SANSON Andrea, 王蕾, 陈骏. Zn(NCN)单轴的负热膨胀性及机理研究[J]. 高等学校化学学报, 2022, 43(6): 20220124. |
| [15] | 任娜娜, 薛洁, 王治钒, 姚晓霞, 王繁. 热力学数据对1, 3-丁二烯燃烧特性的影响[J]. 高等学校化学学报, 2022, 43(6): 20220151. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||