

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (9): 1795.doi: 10.7503/cjcu20190205
• Articles:Inorganic Chemistry • Previous Articles Next Articles
WANG Yue,GUO Xiaohong,ZHOU Guangdong,CHENG Tiexin( )
)
Received:2019-04-08
Online:2019-09-10
Published:2019-07-19
Contact:
CHENG Tiexin
E-mail:ctx@jlu.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Yue, GUO Xiaohong, ZHOU Guangdong, CHENG Tiexin. Effect of Alkyl Benzene Sulfonate Surfactant on Morphology and Structure of Calcium Silicate Hydrate †[J]. Chem. J. Chinese Universities, 2019, 40(9): 1795.
| Group | Name of the product | cSDBS/(mmol·L-1) | Reaction time/d | Stirring or not | ||
|---|---|---|---|---|---|---|
| 1 | #Si4 | 5.0 | 4.0 | 2.0 | 1 | Yes |
| #Si5 | 5.0 | 5.0 | 2.0 | 1 | Yes | |
| #Si6 | 5.0 | 6.0 | 2.0 | 1 | Yes | |
| #Si8 | 5.0 | 8.0 | 2.0 | 1 | Yes | |
| #Si10 | 5.0 | 10.0 | 2.0 | 1 | Yes | |
| 2 | #SDBS2 | 0.005 | 12.5 | 2.0 | 10 | Yes |
| #SDBS4 | 0.005 | 12.5 | 4.0 | 10 | Yes | |
| #SDBS6 | 0.005 | 12.5 | 6.0 | 10 | Yes | |
| #SDBS10 | 0.005 | 12.5 | 10.0 | 10 | Yes | |
| 3 | #t1 | 5.0 | 5.0 | 2.0 | 1 | Yes |
| #t2 | 5.0 | 5.0 | 2.0 | 2 | Yes | |
| #t4 | 5.0 | 5.0 | 2.0 | 4 | Yes | |
| #t20 | 5.0 | 5.0 | 2.0 | 20 | Yes | |
| 4 | #r1 | 5.0 | 5.0 | 2.0 | 1 | Yes |
| #r2 | 5.0 | 5.0 | 2.0 | 1 | No | |
| #r3 | 5.0 | 5.0 | 3.0 | 1 | Yes | |
| #r4 | 5.0 | 5.0 | 3.0 | 1 | No |
| Group | Name of the product | cSDBS/(mmol·L-1) | Reaction time/d | Stirring or not | ||
|---|---|---|---|---|---|---|
| 1 | #Si4 | 5.0 | 4.0 | 2.0 | 1 | Yes |
| #Si5 | 5.0 | 5.0 | 2.0 | 1 | Yes | |
| #Si6 | 5.0 | 6.0 | 2.0 | 1 | Yes | |
| #Si8 | 5.0 | 8.0 | 2.0 | 1 | Yes | |
| #Si10 | 5.0 | 10.0 | 2.0 | 1 | Yes | |
| 2 | #SDBS2 | 0.005 | 12.5 | 2.0 | 10 | Yes |
| #SDBS4 | 0.005 | 12.5 | 4.0 | 10 | Yes | |
| #SDBS6 | 0.005 | 12.5 | 6.0 | 10 | Yes | |
| #SDBS10 | 0.005 | 12.5 | 10.0 | 10 | Yes | |
| 3 | #t1 | 5.0 | 5.0 | 2.0 | 1 | Yes |
| #t2 | 5.0 | 5.0 | 2.0 | 2 | Yes | |
| #t4 | 5.0 | 5.0 | 2.0 | 4 | Yes | |
| #t20 | 5.0 | 5.0 | 2.0 | 20 | Yes | |
| 4 | #r1 | 5.0 | 5.0 | 2.0 | 1 | Yes |
| #r2 | 5.0 | 5.0 | 2.0 | 1 | No | |
| #r3 | 5.0 | 5.0 | 3.0 | 1 | Yes | |
| #r4 | 5.0 | 5.0 | 3.0 | 1 | No |
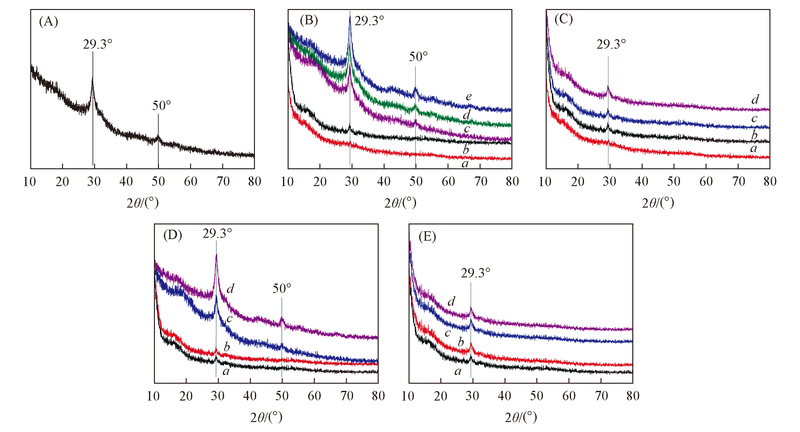
Fig.1 XRD patterns of CSH prepared under different experimental conditions (A) Standard pattern of CSH; (B) a. #Si4, b. #Si5, c. #Si6, d. #Si8, e. #Si10; (C) a. #SDBS2, b. #SDBS4, c. #SDBS6, d. #SDBS10; (D) a. #t1, b. #t2, c. #t4, d. #t20; (E) a. #r1, b. #r2, c. #r3, d. #r4.
| Assignment | Assignment | ||
|---|---|---|---|
| 455-463 | δ(Si-O-Si) | 816-818 | ν(Si-O)Q1 |
| 652-667 | νs(Si-O-Si) | 970-984 | ν(Si-O)Q2 |
| Assignment | Assignment | ||
|---|---|---|---|
| 455-463 | δ(Si-O-Si) | 816-818 | ν(Si-O)Q1 |
| 652-667 | νs(Si-O-Si) | 970-984 | ν(Si-O)Q2 |
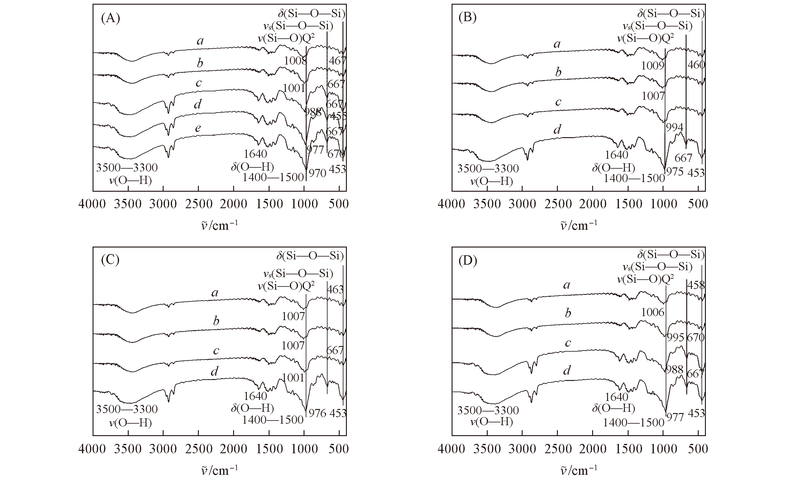
Fig.3 FTIR spectra of CSH under different experimental conditions (A) a. #Si4, b. #Si5, c. #Si6, d. #Si8, e. #Si10; (B) a. #SDBS2, b. #SDBS4, c. #SDBS6, d. #SDBS10; (C) a. #t1, b. #t2, c. #t4, d. #t20; (D) a. #r1, b. #r2, c. #r3, d. #r4.
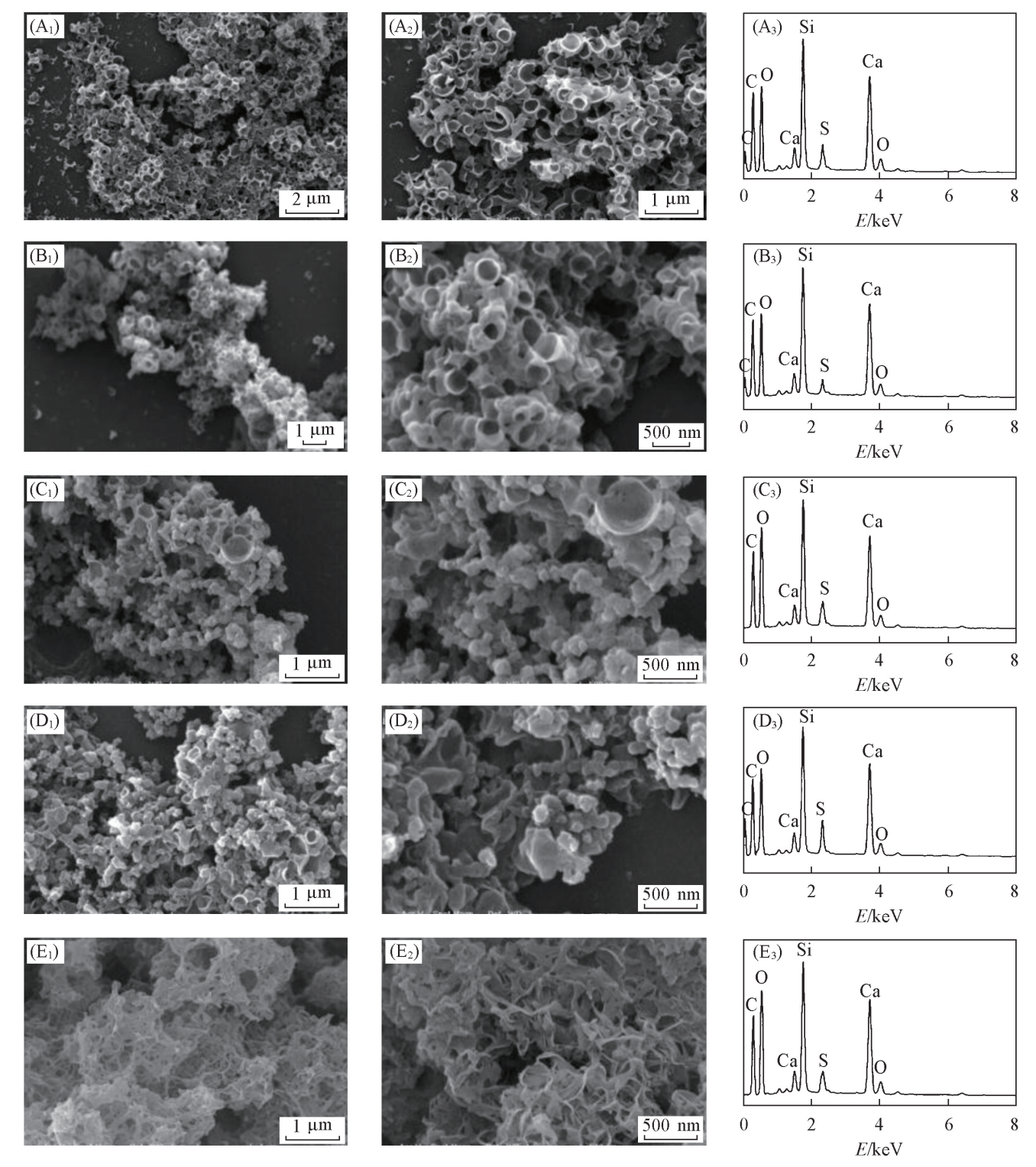
Fig.4 SEM images[(A1-E1), (A2-E2)] and EDS patterns(A3-E3) of CSH prepared with different Na2SiO3·9H2O concentrations (A1)-(A3) #Si4; (B1)-(B3) #Si5; (C1)-(C3) #Si6; (D1)-(D3) #Si8; (E1)-(E3) #Si10.
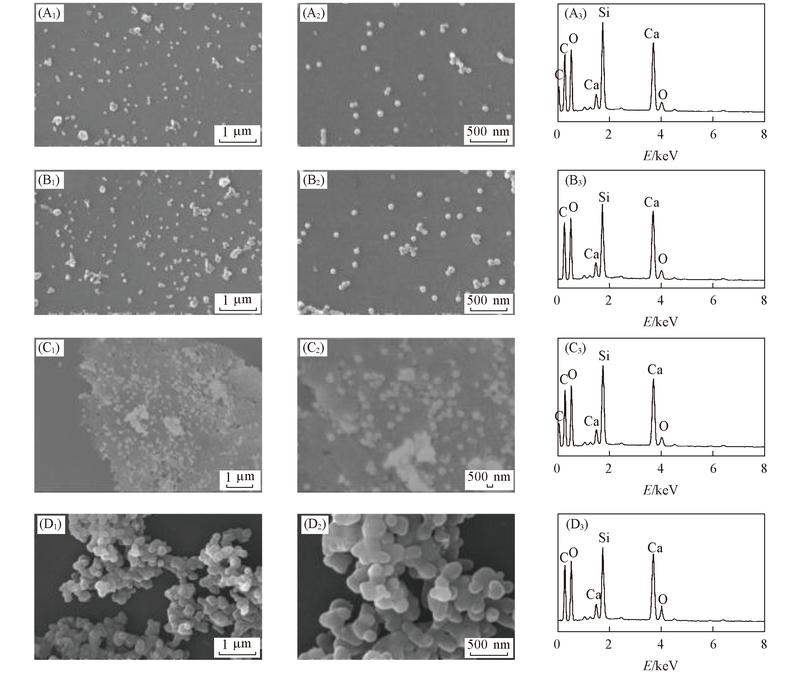
Fig.5 SEM images[(A1-D1), (A2-D2)] and EDS patterns(A3-D3) of CSH prepared with different SDBS concentrations (A1)-(A3) #SDBS2; (B1)-(B3) #SDBS4; (C1)-(C3) #SDBS6; (D1)-(D3) #SDBS10.
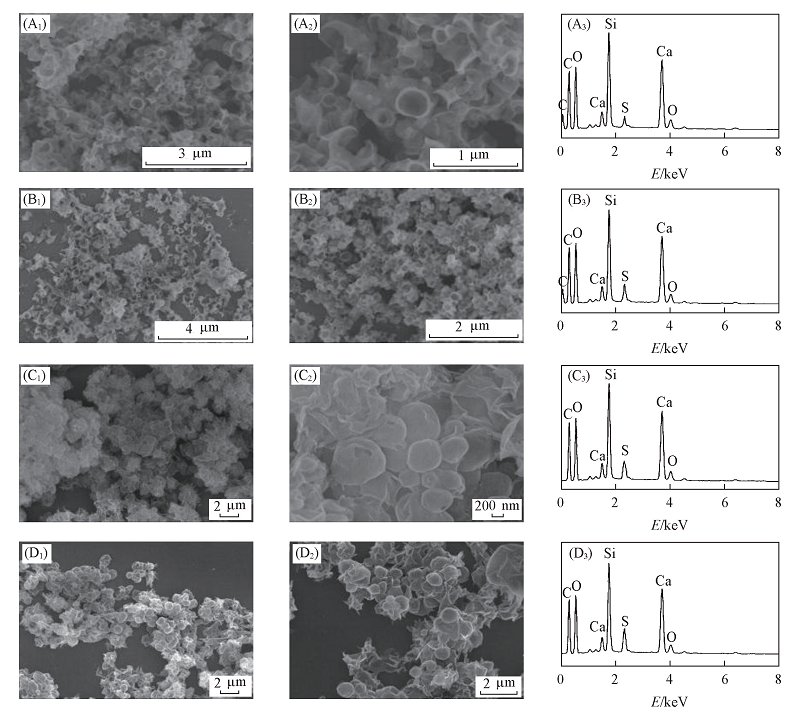
Fig.7 SEM images[(A1-D1), (A2-D2)] and EDS patterns(A3-D3) of CSH prepared with stirring or not (A1)-(A3) #r1; (B1)-(B3) #r2; (C1)-(C3) #r3; (D1)-(D3) #r4.
| [1] | Liu X. W., Huang S. S., Qiu G. Z., , Conservation and Utilization of Mineral Resources, 2001, 21( 1) , 20-24 |
| 刘晓文, 黄圣生, 邱冠周. , 矿产保护与利用,2001, 21( 1) , 20-24) | |
| [2] | Fan E. R., Conservation and Utilization of Mineral Resources , 1996, 16( 4), 17-20 |
| (范恩荣. 矿产保护与利用, 1996, 16( 4), 17-20) | |
| [3] | Adylov G. T., Voronov G. V., Gornostaeva S. A ., Refract. Ind Ceram, 2002, 43( 11/12), 359-361 |
| [4] | Sawyer A. N., Nikonov S. Y., Pancio A. K., J. Endodont ., 2012, 38( 5), 680-683 |
| [5] | Lin K. L., Chang J., Zeng Y., ., Mater Lett, 2004, 58( 15), 2109-2113 |
| [6] | Ma M. G., Zhu J. F., Sun R. C., Chen F., Zhu Y. J ., Mater Lett, 2011, 65( 3), 424-426 |
| [7] | Ginebra M. P., Traykova T., Planell J. A ., J. Controlled Release 2006, 113( 2), 102-110 |
| [8] | Sokolova V., Epple M ., Angew. Chem. Int Ed, 2008, 47( 8), 1382-1395 |
| [9] | Lin K., Chang J., Cheng R ., Acta Biomater 2007, 3( 2), 271-276 |
| [10] | Chang J ., Biomaterials, 2008, 29( 17), 2588-2596 |
| [11] | Pei L. Z., Yang L. J., Yang Y., Fan C. G., Yin W. Y., Chen J., Zhang Q. F ., Mater Charact, 2010, 61( 11), 1281-1285 |
| [12] | Lin K., Chang J., Lu J ., Mater Lett, 2006, 60( 24), 3007-3010 |
| [13] | Zhang M., Chang J ., Ultrason Sonochem, 2010, 17( 5), 789-792 |
| [14] | Wu J., Zhu Y. J., Cao S. W ., Adv Mater, 2010, 22( 6), 749-753 |
| [15] | Ma M. Y., Zhu Y. J., Li L., Cao S. W ., J. Mater Chem, 2008, 18( 23), 2722-2727 |
| [16] | Li X., Chang J ., Chem Lett, 2004, 33( 11), 1458-1459 |
| [17] | Pei L. Z., Pei Y. Q., Li D. K., Yang Y., Yuan C. Z ., Micro&nanosystems, 2011, 3( 2), 161-165 |
| [18] | Baciu D., Simitzis J ., J. Optoelectron. Adv M, 2007, 9( 11), 3320-3324 |
| [19] | Zhang L., Zhao C. M., Jiang Y. L ., Colloids Surf.A 2018, 540( 249-255 |
| [20] | Sui G. H., Cheng Y. Y., Chen Z. M., Wei Q. L., Wang X. F., Yang X. M., Wang Z. C., ., Chem. J. Chinese University 2019, 40( 2), 224-229 |
| (隋光辉, 程岩岩, 陈志敏, 魏庆玲, 王晓峰, 杨晓敏, 王子忱. 高等学校化学学报, 2019, 40(2), 224-229) | |
| [21] | Cheng Y. Y., Sui G. H., Chen Z. M., Liu H., Duan Y. J., Wang X. F., Yang X. M., Wang Z. C., ., Chem. J. Chinese University 2018, 39( 6), 1132-1137 |
| (程岩岩, 隋光辉, 陈志敏, 刘欢, 段亚军, 王晓峰, 杨晓敏, 王子忱. 高等学校化学学报, 2018, 39(6), 1132-1137) | |
| [22] | Jeffery J. W., Bernal J. D., Taylor H. F. W ., Mag. Concrete Res, 2015, 4( 11), 49-54 |
| [23] | Richardson I. G., Groves G. W ., Cem. Concr Res, 1993, 23( 1), 131-138 |
| [24] | Cong X., Kirkpatrick R. J ., Adv. Cem. Based Mater, 1996, 3( 3), 144-156 |
| [25] | Grutzeck M. W., Kwan S., Thompson J. L. , ., J. Mater. Sci. Lett., 1999, 18( 3), 217-220 |
| [26] | Chen J. J., Thomas J. J., Taylor H. F. W., Jennings H. M ., Cem. Concr Res, 2004, 34( 9), 1499-1519 |
| [27] | Nonat A ., Cem. Concr Res, 2004, 34( 9), 1521-1528 |
| [28] | Kantro D. L., Brunauer S., Weise C. H ., J. Phys Chem, 1962, 66( 10), 1804-1809 |
| [29] | Skibsted J., Hall C ., Cem. Concr Res, 2008, 38( 2), 205-225 |
| [30] | Yu P., Kirkpatrick R. J., Poe B ., J. Am. Ceram Soc, 1999, 82( 3), 742-748 |
| [31] | Peng L ., J. Am. Ceram Soc, 2008, 91( 3), 955-964 |
| [1] | ZHANG Zhibo, SHANG Han, XU Wenxuan, HAN Guangdong, CUI Jinsheng, YANG Haoran, LI Ruixin, ZHANG Shenghui, XU Huan. Self-Assembly of Graphene Oxide at Poly(3-hydroxybutyrate) Microparticles Toward High-performance Intercalated Nanocomposites [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210566. |
| [2] | XU Huan, KE Lyu, TANG Mengke, SHANG Han, XU Wenxuan, ZHANG Zilin, FU Yanan, HAN Guangdong, CUI Jinsheng, YANG Haoran, GAO Jiefeng, ZHANG Shenghui, HE Xinjian. In⁃situ Liquid Exfoliation of Montmorillonite Nanosheets in Poly(lactic acid) to Resist Oxygen Permeation [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220316. |
| [3] | WANG Ye, ZHANG Xiaosi, SUN Lijing, LI Bing, LIU Lin, YANG Miao, TIAN Peng, LIU Zhongyi, LIU Zhongmin. Morphology Control of SAPO Molecular Sieves under the Assistance of Organosilane [J]. Chem. J. Chinese Universities, 2021, 42(3): 683. |
| [4] | LU Man,SONG Chunmei,WAN Bo. Thixotropic Behavior of Hydrophobically Modified Ethoxylated Urethane-thickened Waterborne Latex † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1108. |
| [5] | WU Fengren,LIU Yongjia,LU Xuemin,ZHU Bangshang. Controllable Preparation of Polydopamine Modified Gold Nanoflowers and Its Application in Photothermal Therapy † [J]. Chem. J. Chinese Universities, 2020, 41(3): 465. |
| [6] | SHI Xiaoyu, WANG Songmeng, LIU Lingyan, CHANG Weixing, LI Jing. Controllable Synthesis of Amphiphilic Block Copolymer PMnEOS-b-PAA and co-Assembly Morphologies of PMDEOS-b-PAA/PS-b-PAA [J]. Chem. J. Chinese Universities, 2020, 41(11): 2545. |
| [7] | REN Wei, TIAN Ye, XING Lingli, YANG Yuexi, DING Tong, LI Xingang. K Promoted Nanosheets-like Hydrotalcite-derived CoAlO Metal Oxides for Catalytic Soot Combustion [J]. Chem. J. Chinese Universities, 2019, 40(8): 1670. |
| [8] | YANG Jinge, LI Yujie, LU Di, CHEN Yufang, SUN Weiwei, ZHENG Chunman. Morphology Control and Lithium Storage Performance of Micro/nano Li-rich Cathode Material† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1495. |
| [9] | HAN Ning, XIE Ruihong, WANG Xuena, LIU Shuxia. Morphology Modulation and Photocatalytic Performance of Microcrystal of Silver Salt of Ti-substituted Keggin-type Polyoxotungstate† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1378. |
| [10] | ZHANG Shupeng, CHENG Youxing, REN Lei, WEN Kai, LÜ Xiaolin, YE Shefang, ZHOU Xi. Reparation and Photothermal Properties of Prussian Blue Nanoparticles with Different Morphologies† [J]. Chem. J. Chinese Universities, 2018, 39(2): 359. |
| [11] | LI Caixin, LIANG Xiaorong, GU Ju. Preparation and Characterization of Bagasse Nanocellulose† [J]. Chem. J. Chinese Universities, 2017, 38(7): 1286. |
| [12] | ZHAO Fang, WANG Jianjiang, XU Baocai, LIU Jiawei, GAO Haitao. Electrospinning Fabrication and Microwave Absorption Properties of Lithium Zinc Ferrite Micro/Nanofibers [J]. Chem. J. Chinese Universities, 2017, 38(6): 922. |
| [13] | LIU Jiawei, WANG Jianjiang, ZHAO Fang, XU Baocai. Electrospinning Preparation and Infrared-emissivity Properties of La0.67Ba0.33MnO3 Micro/nanofibers [J]. Chem. J. Chinese Universities, 2017, 38(6): 929. |
| [14] | ZHANG Yujian, ZHAI Xuli, FU Kaimei, JIANG Tao. Synthesis of Nano ZSM-23 Zeolites with Low L/D Value Morphology and Their Hydroisomerization Performance† [J]. Chem. J. Chinese Universities, 2017, 38(2): 231. |
| [15] | LI Ru, YU Liangmin, YAN Xuefeng, JIANG Tao. Morphology-controlled Preparation and Photocatalytic Properties of Cu2O/ZnO Microstructures† [J]. Chem. J. Chinese Universities, 2017, 38(2): 267. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||