

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (2): 267.doi: 10.7503/cjcu20160502
• Physical Chemistry • Previous Articles Next Articles
LI Ru1,2, YU Liangmin1, YAN Xuefeng1,*( ), JIANG Tao3
), JIANG Tao3
Received:2016-07-13
Online:2017-02-10
Published:2017-01-16
Contact:
YAN Xuefeng
E-mail:yanxuefeng@ouc.edu.cn
Supported by:CLC Number:
TrendMD:
LI Ru, YU Liangmin, YAN Xuefeng, JIANG Tao. Morphology-controlled Preparation and Photocatalytic Properties of Cu2O/ZnO Microstructures†[J]. Chem. J. Chinese Universities, 2017, 38(2): 267.
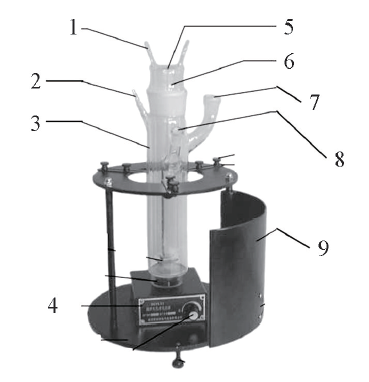
Fig.1 Experimental setup used for photocatalytic experiments1. Cooling water nozzle; 2. snorkel; 3. reactor; 4. magnetic force stirrer; 5. insert type long Hg lamp; 6. quartz cool trap; 7. condensation tube; 8. sample tap; 9. reactor stand.
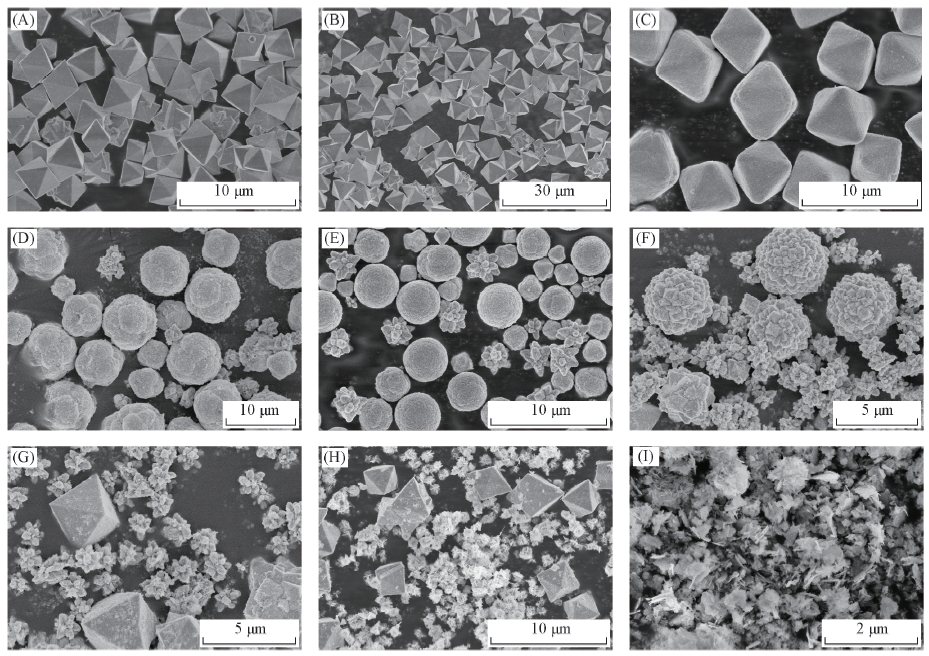
Fig.2 SEM images of Cu2O/ZnO photocatalysts synthesized at various [Cu2+]/[Zn2+] ratios[Cu2+]/[Zn2+]: (A) 1:0.025; (B) 1:0.05; (C) 1:0.15; (D) 1:0.18; (E) 1:0.25; (F) 1:0.30; (G) 1:0.40;(H) 1:0.50; (I) 1:1.00.
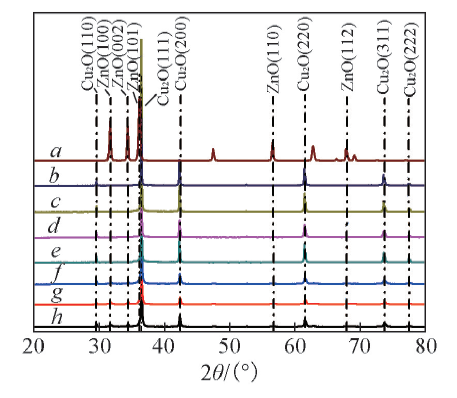
Fig.4 XRD patterns of the pristine Cu2O and various Cu2O/ZnOa. Zn2O; b. Cu2O; c. Cu2O/ZnO-1:0.025; d. Cu2O/ZnO-1:0.05; e. Cu2O/ZnO-1:0.015; f. Cu2O/ZnO-1:0.25; g. Cu2O/ZnO-1:0.50; h. Cu2O/ZnO-1:1.10.
| Sample | β | 2θ/(°) | Crystallite size/nm | Δd/d | SBET/(m2·g-1) | Degradation rate(%) | R2 |
|---|---|---|---|---|---|---|---|
| Cu2O | 0.0029 | 36.46 | 49.82 | 0.0022 | 37.3±0.1 | 0.989 | |
| ZnO | 38.0±0.2 | 0.955 | |||||
| Cu2O/ZnO-1:0.025 | 0.0025 | 36.48 | 57.79 | 0.0019 | 2.12 | 33.2±0.1 | 0.969 |
| Cu2O/ZnO-1:0.05 | 0.0027 | 36.48 | 53.51 | 0.0020 | 7.61 | ||
| Cu2O/ZnO-1:0.15 | 0.0028 | 36.55 | 51.61 | 0.0021 | |||
| Cu2O/ZnO-1:0.25 | 0.0032 | 36.56 | 45.16 | 0.0024 | 41.4±0.1 | 0.936 | |
| Cu2O/ZnO-1:0.30 | 0.0033 | 36.55 | 43.79 | 0.0025 | 6.66 | 41.6±0.1 | 0.990 |
| Cu2O/ZnO-1:0.40 | 0.0034 | 36.54 | 42.50 | 0.0026 | 43.61 | 55.0±0.2 | 0.831 |
| Cu2O/ZnO-1:0.50 | 0.0037 | 36.53 | 39.05 | 0.0028 | 62.11 | 77.5±0.1 | 0.806 |
| Cu2O/ZnO-1:0.80 | 0.0035 | 36.51 | 41.28 | 0.0027 | |||
| Cu2O/ZnO-1:1.00 | 0.0025 | 36.48 | 57.79 | 0.0019 | 61.54 | 30.5±0.2 | 0.911 |
Table 1 Structural parameters extracted from XRD patterns and photocatalytic results of different photocatalysts
| Sample | β | 2θ/(°) | Crystallite size/nm | Δd/d | SBET/(m2·g-1) | Degradation rate(%) | R2 |
|---|---|---|---|---|---|---|---|
| Cu2O | 0.0029 | 36.46 | 49.82 | 0.0022 | 37.3±0.1 | 0.989 | |
| ZnO | 38.0±0.2 | 0.955 | |||||
| Cu2O/ZnO-1:0.025 | 0.0025 | 36.48 | 57.79 | 0.0019 | 2.12 | 33.2±0.1 | 0.969 |
| Cu2O/ZnO-1:0.05 | 0.0027 | 36.48 | 53.51 | 0.0020 | 7.61 | ||
| Cu2O/ZnO-1:0.15 | 0.0028 | 36.55 | 51.61 | 0.0021 | |||
| Cu2O/ZnO-1:0.25 | 0.0032 | 36.56 | 45.16 | 0.0024 | 41.4±0.1 | 0.936 | |
| Cu2O/ZnO-1:0.30 | 0.0033 | 36.55 | 43.79 | 0.0025 | 6.66 | 41.6±0.1 | 0.990 |
| Cu2O/ZnO-1:0.40 | 0.0034 | 36.54 | 42.50 | 0.0026 | 43.61 | 55.0±0.2 | 0.831 |
| Cu2O/ZnO-1:0.50 | 0.0037 | 36.53 | 39.05 | 0.0028 | 62.11 | 77.5±0.1 | 0.806 |
| Cu2O/ZnO-1:0.80 | 0.0035 | 36.51 | 41.28 | 0.0027 | |||
| Cu2O/ZnO-1:1.00 | 0.0025 | 36.48 | 57.79 | 0.0019 | 61.54 | 30.5±0.2 | 0.911 |
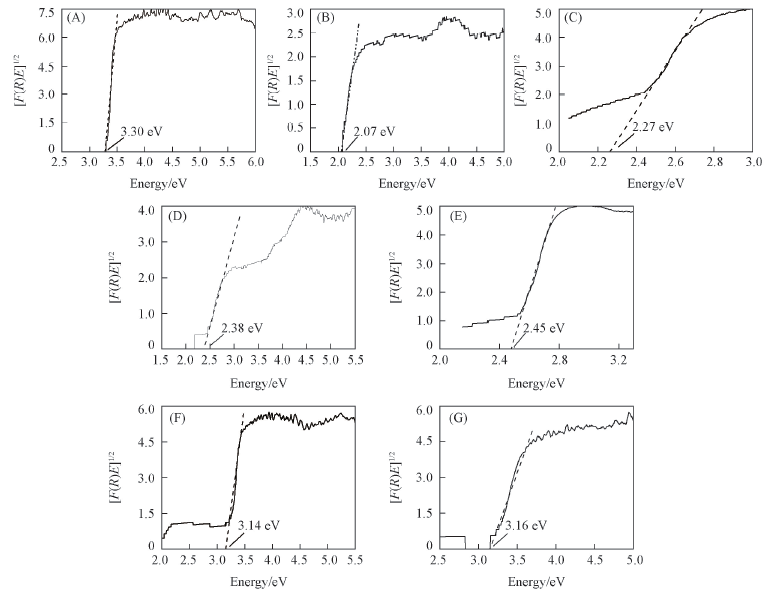
Fig.7 Determination of the band gap energy of pure ZnO(A), pure Cu2O(B), and Cu2O/ZnO synthesized at [Cu2+]/[Zn2+]=1:0.05(C), 1:0.15(D), 1:0.30(E), 1:0.50(F), 1:1.0(G)
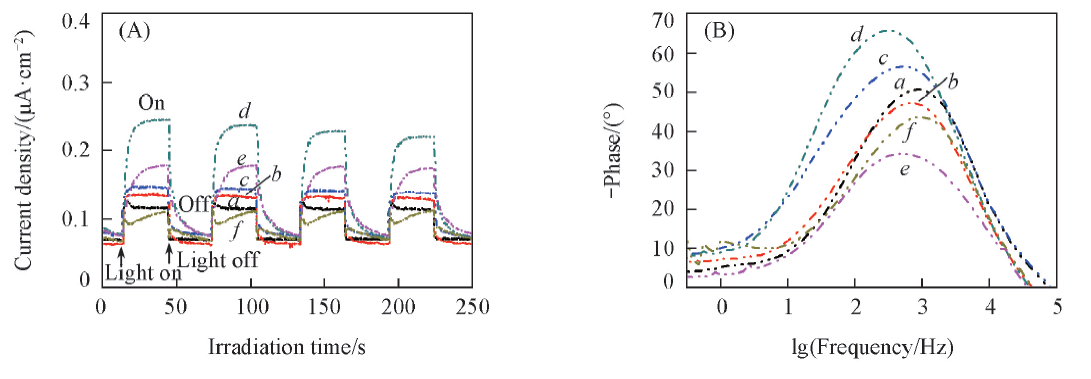
Fig.11 Transient photocurrent responses(A) and Bode EIS plots(B) of pseudo cells with different Cu2O/ZnO photoanodes a. Cu2O/ZnO-1:0.25; b. Cu2O/ZnO-1:0.30; c. Cu2O/ZnO-1:0.40; d. Cu2O/ZnO-1:0.50; e. Cu2O/ZnO-1:0.80; f. Cu2O/ZnO-1:1.00.
| [1] | Schüler E., Gustavsson A. K., Hertenberger S., Sattler K., Sol. Energy,2013, 96, 220—226 |
| [2] | Li X. H., Chen G. Y., Yang L. B., Jin Z., Liu J. H., Adv. Funct. Mater., 2010, 20, 2815—2824 |
| [3] | Luo Z. W., Jiang H., Li D., Hu L. Z., Geng W. H., Wei P., Ouyang P. K., RSC Adv., 2014, 4, 17797—17804 |
| [4] | Diab M., Moshofsky B., Plante I. J. L., Mokari T., J. Mater. Chem., 2011, 21, 11626—11630 |
| [5] | Zhao H. Y., Wang Y. F., Zeng J. H., Cryst. Growth Des., 2008, 8, 3731—3734 |
| [6] | Younsi M., Aider A., Bouguelia A., Trari M., Sol. Energy,2005, 78, 574—580 |
| [7] | Siegfried M. J., Choi K. S., Adv. Mater., 2004, 16, 1743—1746 |
| [8] | Xu H., Wang W., Zhu W., J. Phys. Chem. B,2006, 110, 13829—13834 |
| [9] | White B., Yin M., Hall A., Le D., Stolbov S., Rahman T., Turro N., O’Brien S., Nano Lett., 2006, 6, 2095—2098 |
| [10] | Sui Y. M., Zeng Y., Fu L. L., Zheng W. T., Li D. M., Liu B. B., Zou B., RSC Adv., 2013, 3, 18651—18660 |
| [11] | Zhang H., Zhu Q., Zhang Y., Wang Y., Zhao L., Yu B., Adv. Funct. Mater., 2007, 17, 2766—2771 |
| [12] | Paracchino A., Laporte V., Sivula K., Grätzel M., Thimsen E., Nat. Mater., 2011, 10, 456—461 |
| [13] | Sivagami K., Krishna R. R., Swaminathan T., Sol. Energy,2014, 103, 488—493 |
| [14] | Wu S. X., Yin Z. Y., He Q. Y., Lu G., Zhou X. Z., Zhang H., J. Mater. Chem., 2011, 21, 3467—3470 |
| [15] | Han Z. Z., Ren L. L, Cui Z. H., Chen C. Q., Pan H. B., Chen J. Z., Appl. Catal. B,2012, 126, 298—305 |
| [16] | Morales-Flores N., Pal U., Mora E. S., Appl. Catal. A,2011, 394, 269—275 |
| [17] | Jing L. Q., Wang B. Q., Xin B. F., Li S. D., Shi K. Y., Cai W. M., Fu H. G., J. Solid State Chem., 2004, 177, 4221—4227 |
| [18] | Forzani E. S., Lu D. L., Leright M. J., Aguilar A. D., Tsow F., Iglesias R. A., Zhang Q., Lu J., Li J. H., Tao N. J., J. Am. Chem. Soc., 2009, 131, 1390—1391 |
| [19] | Li Q. H., Jin X., Yang X. W., Chen C. Y., Chen Z. H., Qin Y. C., Wei T. H, Sun W. F., Appl. Catal., B,2015, 162, 524—531 |
| [20] | Jin X., Sun W. F., Chen Z. H., Wei T. H., Chen C. Y., He X. D., Yuan Y. B., Li Y., Li Q. H., ACS Appl. Mater. Interfaces,2014, 6, 8771—8781 |
| [21] | Li R., Yu L. M., Yan X. F., Tang Q. W., RSC Adv., 2015, 5, 11917—11924 |
| [22] | Jing L. Q., Sun X. J., Xin B. F., Wang B. Q., Cai W. M., Fu H. G., J. Solid State Chem., 2004, 177, 3375—3382 |
| [23] | Zhang Q. H., Gao L., Guo J. K., Appl. Catal. B-Environ., 2000, 26, 207—215 |
| [24] | Lin L., Chai Y. C., Yang Y. C., Wang X. He D. N., Tang Q. W., Ghoshroy S., Int. J. Hydrogen Energy,2013, 38, 2634—2640 |
| [25] | Kortum G. Reflectance Spectroscopy; Springer-Verlag: Berlin, 1969, 15—21 |
| [26] | Zhang W.G., Halasyamani P. S., Cryst. Growth Des., 2012, 12, 2127—2132 |
| [27] | Wodka D., Bielanska E., Socha R.P., Elzbeciak-Wodka M., Gurgul J., Nowak P., Warszyński P., Kumakiri I., ACS Appl. Mater. Interfaces, 2010, 2, 1945—1955 |
| [28] | Xu C., Cao L. X., Su G., Liu W., Liu H., Yu Y. Q., Qu X. F., J. Hazard. Mater., 2010, 176, 807—813 |
| [29] | Li R., Yan X. F., Yu L. M., Zhang Z. M., Tang Q. W., Pan Y. P., Cryst. Eng. Comm., 2013, 15, 10049—10058 |
| [30] | Huang L., Peng F., Wang H. J., Yu H., Li Z., Catal. Commun., 2009, 10, 1839—1843 |
| [31] | Ullah R., Dutta J., J. Hazard. Mater., 2008, 156, 194—200 |
| [32] | Li D. P., Dai K., Lv J. L., Lu L. H., Liang C. H., Zhu G. P., Materials Letters,2015, 150, 48—51 |
| [33] | Suib S. L., Segal S. R., Chem. Mater., 1997, 9, 2526—2532 |
| [34] | Cai H. Y., Chen X. X., Li Q. H., He B. L., Tang Q. W., Appl. Surf. Sci., 2013, 284, 837—842 |
| [35] | Lin L., Yang Y. C., Men L., Wang X. He D. N., Chai Y. C., Zhao B., Ghoshroy S., Tang Q. W., Nanoscale,2013, 5, 588—593 |
| [1] | SONG Yingying, HUANG Lin, LI Qingsen, CHEN Limiao. Preparation of CuO/BiVO4 Photocatalyst and Research on Carbon Dioxide Reduction [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220126. |
| [2] | YANG Xiaomei, WU Qiang, GUO Ru, YE Kaibo, XUE Ping, WANG Xiaozhong, LAI Xiaoyong. Ordered Mesoporous NiS-loaded CdS with Ultrathin Frameworks for Efficient Photocatalytic H2 Production [J]. Chem. J. Chinese Universities, 2021, 42(5): 1581. |
| [3] | LI Shanshan, ZHAO Wenjuan, LI Hui, FANG Qianrong. A Photoresponsive Azobenzene-functionalized Covalent Organic Framework † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1384. |
| [4] | MA Xiangying, LIAO Yanjun, QIN Fanghong, YIN Yuanhao, HUANG Zaiyin, CHEN Qifeng. Study on the Photocatalytic Performance of Carbon Doped g-C3N4 Based on in situ Photomicrocalorimeter-fluorescence Spectrometry [J]. Chem. J. Chinese Universities, 2020, 41(11): 2526. |
| [5] | ZHAO Mengxin, MENG Zhe, LI Heping, MA Zongqin, ZHAN Haijuan, LIU Wanyi. Photodegradation of Antibiotic in Environmental Water by Graphene Oxide Modulation Bismuth Molybdate Under Visible Light Irradiation [J]. Chem. J. Chinese Universities, 2020, 41(11): 2479. |
| [6] | HE Pengchen,ZHOU Jian,ZHOU Awu,DOU Yibo,LI Jianrong. MOFs-Based Materials for Photocatalytic CO2 Reduction† [J]. Chem. J. Chinese Universities, 2019, 40(5): 855. |
| [7] | GAN Lu,DONG Yongchun. Photocatalytic Performance of Fe-complexes Prepared Using Cotton Fiber Modified with Different Dicarboxylic Acids † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2205. |
| [8] | ZHANG Jing,DONG Yuming,LIU Xiang,LI Hexing. Synthesis and Photocatalytic Activity of Z-Scheme Photocatalyst Sb2WO6/g-C3N4 † [J]. Chem. J. Chinese Universities, 2019, 40(1): 123. |
| [9] | CONG Rimin, YU Huaiqing, LUO Yunjun, LI Jiao, WANG Weiwei, LI Qiuhong, SUN Wuzhu, SI Weimeng, ZHANG Hua. Synthesis and Properties of Bi25FeO40/α-Fe2O3 Composite Nanoparticle Photocatalysts† [J]. Chem. J. Chinese Universities, 2018, 39(4): 629. |
| [10] | GAO Xiaoming, DAI Yuan, FEI Jiao, ZHANG Yu, FU Feng. Synthesis of n-p Heterojunction BiOBr/CdS Composites with Enhanced Photocatalytic Properties† [J]. Chem. J. Chinese Universities, 2017, 38(7): 1249. |
| [11] | ZHANG Chunlei, HUANG Danya, SUN Minghui, OUYANG Yiting, WANG Chao, LI Xiaoyun, CHEN Lihua, SU Baolian. Promoting Effect of Nonmetal Ion Doping and Hierarchically 3D Dendrimeric Architecture for Visible-light-active Mesoporous TiO2 Photocatalyst† [J]. Chem. J. Chinese Universities, 2017, 38(3): 471. |
| [12] | CHEN Ying, ZHAO Yu, LI Jing, HAN Xingyue. One-step Synthesis of Hydrangea-like BiOCl/Br Solid Solution Photocatalyst with Co-template Method† [J]. Chem. J. Chinese Universities, 2017, 38(11): 2045. |
| [13] | AN Huiqin, YU Yucai, YAN Lin, WU Tingting, LI Xiaofeng, HE Xiaoling, ZHAO Lizhi, HUANG Weiping. Synthesis of Highly Dispersed Au Nanoparticles Modified N-Doped TiO2 Nanotubes by the Assist of Lysine and Their Photocatalytic Activity† [J]. Chem. J. Chinese Universities, 2016, 37(11): 2034. |
| [14] | SUN Daihong, LIU Huarong, LI Ruizhe. Preparation and Decontamination Capability of Hollow Zn-Cr Ferrite/Titanium Dioxide Composites† [J]. Chem. J. Chinese Universities, 2016, 37(11): 2050. |
| [15] | LU Yonghong, WU Pingxiao, HUANG Junyi, TRAN Lytuong, ZHU Nengwu, DANG Zhi. Alkaline-assisted Hydrothermal Fabrication of CdZnS with Enhanced Visible-light Photocatalytic Performance† [J]. Chem. J. Chinese Universities, 2015, 36(8): 1563. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||