

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (9): 1886.doi: 10.7503/cjcu20180269
• Articles:Inorganic Chemistry • Previous Articles Next Articles
WANG Dongmei1,*( ), LIU Zihua1, LI Guanghua2, LIU Yunling2, LI Chunxia1,*(
), LIU Zihua1, LI Guanghua2, LIU Yunling2, LI Chunxia1,*( )
)
Received:2018-04-09
Online:2018-07-30
Published:2018-07-30
Contact:
WANG Dongmei,LI Chunxia
E-mail:dmwang@zjnu.edu.cn;cxli@zjnu.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Dongmei,LIU Zihua,LI Guanghua,LIU Yunling,LI Chunxia. Synthesis, Structure and Fluorescent Property of Indium-based Bimetallic Metal-organic Frameworks†[J]. Chem. J. Chinese Universities, 2018, 39(9): 1886.
| Complex | 1 | 2 | ||
|---|---|---|---|---|
| Empirical formula | C21H22CuInN9O7 | C13H18Cd0.5In0.5N6.5O6.5 | ||
| Formula weight | 690.84 | 482.95 | ||
| Temperature/K | 293(2) | 293(2) | ||
| Crystal system | Orthorhombic | Orthorhombic | ||
| Space group | Pmma | Imma | ||
| a/nm | 0.72059(14) | 1.8826(4) | ||
| b/nm | 1.8500(4) | 0.73469(15) | ||
| c/nm | 1.5380(3) | 1.5903(3) | ||
| α/(°) | 90 | 90 | ||
| β/(°) | 90 | 90 | ||
| γ/(°) | 90 | 90 | ||
| V/nm3 | 2.0503(7) | 2.1996(8) | ||
| Z | 2 | 4 | ||
| Dc/(Mg·m-3) | 1.119 | 1.074 | ||
| μ/mm-1 | 1.361 | 0.971 | ||
| F(000) | 690 | 968 | ||
| Reflections collected/Unique(Rint) | 19273/2614(0.0695) | 5518/1101(0.0193) | ||
| Goodness-of-fit on F2 | 1.093 | 1.127 | ||
| R1, wR2[I>2σ(I)]* | 0.0402, 0.1263 | 0.0249, 0.0701 | ||
| R1, wR2(all data)* | 0.0529, 0.1329 | 0.0301, 0.0725 | ||
Table 1 Crystal data and structural refinement for complexes 1 and 2
| Complex | 1 | 2 | ||
|---|---|---|---|---|
| Empirical formula | C21H22CuInN9O7 | C13H18Cd0.5In0.5N6.5O6.5 | ||
| Formula weight | 690.84 | 482.95 | ||
| Temperature/K | 293(2) | 293(2) | ||
| Crystal system | Orthorhombic | Orthorhombic | ||
| Space group | Pmma | Imma | ||
| a/nm | 0.72059(14) | 1.8826(4) | ||
| b/nm | 1.8500(4) | 0.73469(15) | ||
| c/nm | 1.5380(3) | 1.5903(3) | ||
| α/(°) | 90 | 90 | ||
| β/(°) | 90 | 90 | ||
| γ/(°) | 90 | 90 | ||
| V/nm3 | 2.0503(7) | 2.1996(8) | ||
| Z | 2 | 4 | ||
| Dc/(Mg·m-3) | 1.119 | 1.074 | ||
| μ/mm-1 | 1.361 | 0.971 | ||
| F(000) | 690 | 968 | ||
| Reflections collected/Unique(Rint) | 19273/2614(0.0695) | 5518/1101(0.0193) | ||
| Goodness-of-fit on F2 | 1.093 | 1.127 | ||
| R1, wR2[I>2σ(I)]* | 0.0402, 0.1263 | 0.0249, 0.0701 | ||
| R1, wR2(all data)* | 0.0529, 0.1329 | 0.0301, 0.0725 | ||
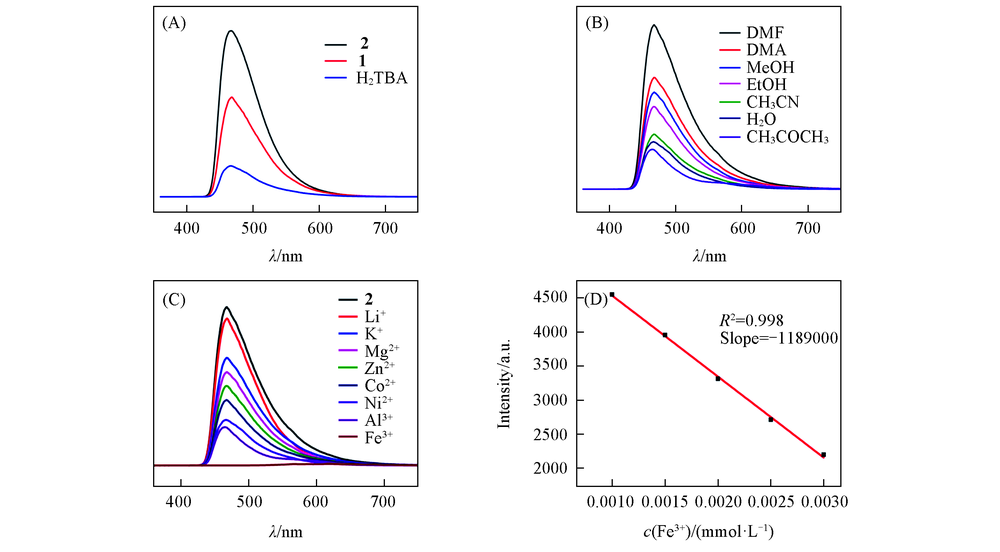
Fig.6 Solid-state emission spectra of H2TBA and complexes 1, 2(A), fluoresence intensity changes of complex 2 in different volatile organic solvents(B) and after addition of different metal ions(C) and linear plot of fluorescence intensity of complex 2 in DMF upon addition of Fe3+(D)
| [1] | He Y., Zhou W., Qian G., Chen B., Chem. Soc. Rev., 2014, 43(16), 5657—5678 |
| [2] | Spanopoulos I., Tsangarakis C., Klontzas E., Tylianakis E., Froudakis G., Adil K., Belmabkhout Y., Eddaoudi M., Trikalitis P. N., J. Am. Chem. Soc., 2016, 138(5), 1568—1574 |
| [3] | Bhatt P. M., Belmabkhout Y., Cadiau A., Adil K., Shekhah O., Shkurenko A., Barbour L. J., Eddaoudi M., J. Am. Chem. Soc., 2016, 138(29), 9301—9307 |
| [4] | Zhai Q. G., Bu X. H., Zhao X., Li D. S., Feng Y. P., Acc. Chem. Res., 2017, 50(2), 407—417 |
| [5] | Kertik A., Wee L. H., Pfannmoller M., Bals S., Martens J. A., Vankelecom I. F. J., Energy Environ. Sci., 2017, 10(11), 2342—2351 |
| [6] | Liu D., Lang J. P., Abrahams B. F., J. Am. Chem. Soc., 2011, 133(29), 11042—11045 |
| [7] | Lang J. P., Xu Q. F., Yuan R. X., Abrahams B. F., Angew. Chem. Int. Ed., 2004, 43(36), 4741—4745 |
| [8] | Yao S., Wang D., Cao Y., Li G., Huo Q., Liu Y., J. Mater. Chem. A, 2015, 3(32), 16627—16632 |
| [9] | Huang Y. B., Liang J., Wang X. S., Cao R., Chem. Soc. Rev., 2017, 46(1), 126—157 |
| [10] | Liu D., Ren Z. G., Li H. X., Lang J. P., Li N. Y., Abrahams B. F., Angew. Chem. Int. Ed., 2010, 49(28), 4767—4770 |
| [11] | Li F. L., Shao Q., Huang X., Lang J. P., Angew. Chem., 2018, 130(7), 1906—1910 |
| [12] | An B., Zhang J., Cheng K., Ji P., Wang C., Lin W., J. Am. Chem. Soc., 2017, 139(10), 3834—3840 |
| [13] | Hu Z., Deibert B. J., Li J., Chem. Soc. Rev., 2014, 43(16), 5815—5840 |
| [14] | Yan B., Acc. Chem. Res., 2017, 50(11), 2789—2798 |
| [15] | Wang S. H., Hu H. Z., Chen C., Ma R. N., Zhang N., Chem. J. Chinese Universities, 2014, 35(10), 2055—2060 |
| (汪淑华, 胡汉珍, 陈超, 马润宁, 张宁. 高等学校化学学报, 2014, 35(10), 2055—2060) | |
| [16] | Wang S. H., Wang P. P., Li P. F., Zhang N., Chen C., Chem. J. Chinese Universities, 2014, 35(12), 2499—2504 |
| (汪淑华, 王萍萍, 李鹏飞, 张宁, 陈超. 高等学校化学学报, 2014, 35(12), 2499—2504) | |
| [17] | Wu Y. N., Zhou M., Li S., Li Z., Li J., Wu B., Li G., Li F., Guan X., Small, 2014, 10(14), 2927—2936 |
| [18] | Gu T. Y., Dai M., Young D. J., Ren Z. G., Lang J. P., Inorg. Chem., 2017, 56(8), 4668—4678 |
| [19] | Chen M. M., Zhou X., Li H. X., Yang X. X., Lang J. P., Cryst. Growth Des., 2015, 15(6), 2753—2760 |
| [20] | Hu F. L., Shi Y. X., Chen H. H., Lang J. P., Dalton Trans., 2015, 44(43), 18795—18803 |
| [21] | Gong W. J., Ren Z. G., Li H. X., Zhang J. G., Lang J. P., Cryst. Growth Des., 2017, 17(2), 870—881 |
| [22] | Ramaswamy P., Wong N. E., Shimizu G. K., Chem. Soc. Rev., 2014, 43(16), 5913—5932 |
| [23] | Hu F. L., Mi Y., Gu Y. Q., Zhu L. G., Yang S. L., Wei H., Lang J. P., Cryst. Eng. Comm., 2013, 15(45), 9553—9561 |
| [24] | Chen Y., Li H. X., Liu D., Liu L. L., Li N. Y., Ye H. Y., Zhang Y., Lang J. P., Cryst. Growth Des., 2008, 8(10), 3810—3816 |
| [25] | Chen Y., Wang Z. O., Ren Z. G., Li H. X., Li D. X., Liu D., Zhang Y., Lang J. P., Cryst. Growth Des., 2009, 9(11), 4963—4968 |
| [26] | Liu D., Ren Z. G., Li H. X., Chen Y., Wang J., Zhang Y., Lang J. P., Cryst. Eng. Comm., 2010, 12(6), 1912—1919 |
| [27] | Férey G., Mellot D. C., Serre C., Millange F., Dutour J., Surble S., Margiolaki I., Science, 2005, 309(5743), 2040—2042 |
| [28] | Cavka J. H., Jakobsen S., Olsbye U., Guillou N., Lamberti C., Bordiga S., Lillerud K. P., J. Am. Chem. Soc., 2008, 130(42), 13850—13851 |
| [29] | Li M., Li D., O’Keeffe M., Yaghi O. M., Chem. Rev., 2014, 114(2), 1343—1370 |
| [30] | Schoedel A., Cairns A. J., Belmabkhout Y., Wojtas L., Mohamed M., Zhang Z., Proserpio D. M., Eddaoudi M., Zaworotko M. J., Angew. Chem. Int. Ed., 2013, 52(10), 2902—2905 |
| [31] | Guillerm V., Kim D., Eubank J. F., Luebke R., Liu X., Adil K., Lah M. S., Eddaoudi M., Chem. Soc. Rev., 2014, 43(16), 6141—6172 |
| [32] | Cook T. R., Zheng Y. R., Stang P. J., Chem. Rev., 2013, 113(1), 734—777 |
| [33] | Dhakshinamoorthy A., Asiri A. M., Garcia H., Catal. Sci. Technol., 2016, 6(14), 5238—5261 |
| [34] | Wang Z., Wang B., Yang Y., Cui Y., Wang Z., Chen B., Qian G., ACS Appl.Mater. Interfaces, 2015, 7(37), 20999—21004 |
| [35] | Park K. C., Seo C., Gupta G., Kim J., Lee C. Y., ACS Appl. Mater. Interfaces, 2017, 9(44), 38670—38677 |
| [36] | Wang D., Zhang L., Li G., Huo Q., Liu Y., RSC Adv., 2015, 5(23), 18087—18091 |
| [37] | Zhang W. H., Ren Z. G., Lang J. P., Chem. Soc. Rev., 2016, 45(18), 4995—5019 |
| [38] | Jiao Y., Morelock C. R., Burtch N. C., Mounfield W. P., Hungerford J. T., Walton K. S., Ind. Eng. Chem. Res., 2015, 54(49), 12408—12414 |
| [39] | Zou R., Li P. Z., Zeng Y. F., Liu J., Zhao R., Duan H., Luo Z., Wang J. G., Zou R., Zhao Y., Small, 2016, 12(17), 2334—2343 |
| [40] | Yang X., Xu Q., Cryst. Growth Des., 2017, 17(4), 1450—1455 |
| [41] | Zheng S. T., Wu T., Chou C., Fuhr A., Feng P., Bu X., J. Am. Chem. Soc., 2012, 134(10), 4517—4520 |
| [42] | Zhai Q. G., Bu X., Mao C., Zhao X., Feng P., J. Am. Chem. Soc., 2016, 138(8), 2524—2527 |
| [43] | Zhang J. W., Hu M. C., Li S. N., Jiang Y. C., Qu P., Zhai Q. G., Chem. Commun., 2018, 54(16), 2012—2015 |
| [44] | Sheldrick G. M., SHELXTL-NT,Version 5.1, Bruker AXS Inc., Madison, WI, 1997 |
| [45] | Blatov V. A., Shevchenko A. P., Proserpio D. M., Cryst. Growth Des., 2014, 14(7), 3576—3586 |
| [1] | LIU Qingqing, WANG Pu, WANG Yongshuai, ZHAO Man, DONG Huanli. Synthesis and Topochemical Polymerization Study of Naphthalene/perylene Imides Substituted Diacetylene Derivatives [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220091. |
| [2] | SHI Naike, ZHANG Ya, SANSON Andrea, WANG Lei, CHEN Jun. Uniaxial Negative Thermal Expansion and Mechanism in Zn(NCN) [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220124. |
| [3] | MA Jianxin, LIU Xiaodong, XU Na, LIU Guocheng, WANG Xiuli. A Multi-functional Zn(II) Coordination Polymer with Luminescence Sensing, Amperometric Sensing, and Dye Adsorption Performance [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210585. |
| [4] | YUE Shengli, WU Guangbao, LI Xing, LI Kang, HUANG Gaosheng, TANG Yi, ZHOU Huiqiong. Research Progress of Quasi-two-dimensional Perovskite Solar Cells [J]. Chem. J. Chinese Universities, 2021, 42(6): 1648. |
| [5] | ZHANG Junying, PENG Wei, CHEN Ziwei, HE Aihua. Effect of the Polymerization Temperature on the Copolymerization of Butadiene and Isoprene Catalyzed by Supported Ziegler-Natta Catalyst [J]. Chem. J. Chinese Universities, 2020, 41(8): 1873. |
| [6] | TIAN Xia,YANG Fuqun,YUAN Wei,ZHAO Lei,YAO Lei,ZHEN Xiaoli,HAN Jianrong,LIU Shouxin. Synthesis, Structure and Recognition Properties of Macrocyclic Crown Ethers with Oxadiazole † [J]. Chem. J. Chinese Universities, 2020, 41(3): 490. |
| [7] | LIU Dongmei,SU Yajing,LI Shanshan,XU Qiwei,LI Xia. Transition Metal Coordination Polymers Constructed by 4-(4-Carboxyphenoxy)isophthalic Acid: Synthesis, Crystal Structure, Fluorescence Sensing and Photocatalysis † [J]. Chem. J. Chinese Universities, 2020, 41(2): 253. |
| [8] | XIAN Guoxuan, YU Yu’e, CHEN Yuqian, WAN Xiaoyu, WANG Suna, LU Jing. Coligand Induced Luminescent Cd-MOFs: Luminescence Enhancement Toward Acetylacetone and Quenching Toward Cr2O72- [J]. Chem. J. Chinese Universities, 2020, 41(12): 2725. |
| [9] | QIN Liulei,LIU Yang,GUAN Xiaoqin,ZHENG Xiaoyuan,ZHANG Ziyu,LIU Zunqi. Synthesis and Switchable Dielectric Properties of an Inorganic-organic Hybrid Complex [H2(DABCO)CuCl4]·H2O † [J]. Chem. J. Chinese Universities, 2020, 41(1): 70. |
| [10] | LI Bing,WANG Xuemin,BAI Fengying,LIU Shuqing. Synthesises, Structures and Antibacterial Activities of a Series of Rare Earth Nitrogen Heterocyclic Complexes† [J]. Chem. J. Chinese Universities, 2019, 40(4): 632. |
| [11] | Baozhen SHI,Shan LI,Dianpeng WANG,Yunzhi ZHOU,Jinyu SUN. Synthesis and Physical Properties of Cobalt-Zinc Hybrid Porous Metal-organic Frameworks † [J]. Chem. J. Chinese Universities, 2019, 40(12): 2443. |
| [12] | SONG Wei, WANG Liqun, ZENG Shuangli, WANG Li, FAN Yong, XU Jianing. In situ Hydrothermal Synthesis, Crystal Structure and Fluorescence Properties of Two Cadmium Coordination Polymers† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1406. |
| [13] |
TIAN Huan, ZHANG Menglong, WANG Lisha, TONG Bihai, ZHAO Zhuo.
Synthesis of 4,13-Dithio Benzene and-18-Crown-6 and Its Selective Extractability on A |
| [14] | LIU Lili, TAI Xishi, LIU Junbo, LI Dan, ZHOU Xiaojing, ZHANG Lijun, WEI Xiaofei. Preparation of Propargylamines Catalyzed by Heterogeneous Catalysts with Double Catalytic Sites† [J]. Chem. J. Chinese Universities, 2018, 39(3): 482. |
| [15] | ZHU Lei,HAN Junyan,CHANG Haizhen,QIU Yuyuan,ZHANG Yanan,PENG Danni,HU Wei,MIAO Shaobin. Different Pathways for the Cyclocondensation Reactions of 1,2-Diamine and 1,2-Diketone† [J]. Chem. J. Chinese Universities, 2018, 39(12): 2686. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||