

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (3): 482.doi: 10.7503/cjcu20170721
• Physical Chemistry • Previous Articles Next Articles
LIU Lili1,*( ), TAI Xishi1,*(
), TAI Xishi1,*( ), LIU Junbo2, LI Dan1, ZHOU Xiaojing1, ZHANG Lijun1, WEI Xiaofei1
), LIU Junbo2, LI Dan1, ZHOU Xiaojing1, ZHANG Lijun1, WEI Xiaofei1
Received:2017-11-10
Online:2018-03-10
Published:2018-01-23
Contact:
LIU Lili,TAI Xishi
E-mail:liulili122@126.com;taixs@wfu.edu.cn
Supported by:CLC Number:
TrendMD:
LIU Lili, TAI Xishi, LIU Junbo, LI Dan, ZHOU Xiaojing, ZHANG Lijun, WEI Xiaofei. Preparation of Propargylamines Catalyzed by Heterogeneous Catalysts with Double Catalytic Sites†[J]. Chem. J. Chinese Universities, 2018, 39(3): 482.
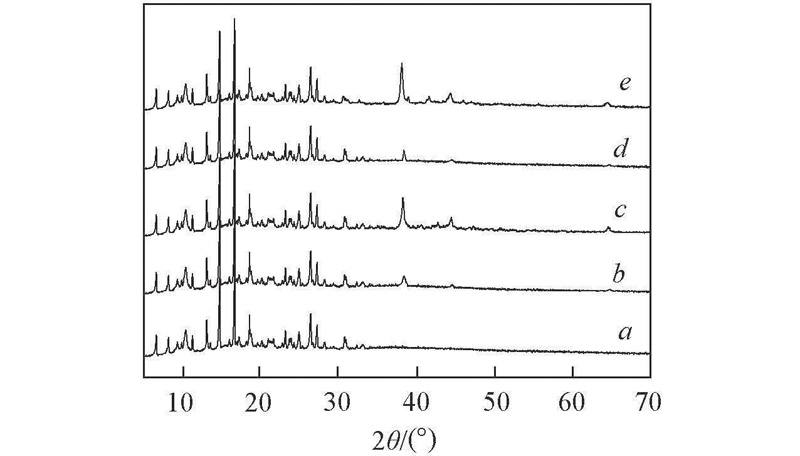
Fig.5 XRD patterns of different samplesa. CP-Ni-NDC; b. fresh 4.49%Au@CP-Ni-NDC; c. 4.49%Au@CP-Ni-NDC after four cycles; d. fresh 3.43%Ag@CP-Ni-NDC; e. 3.43%Ag@CP-Ni-NDC after four cycles.

Fig.6 TEM images of different samples(A) Fresh 4.49%Au@CP-Ni-NDC; (B) 4.49%Au@CP-Ni-NDC after four cycles; (C) fresh 3.43%Ag@CP-Ni-NDC; (D) 3.43%Ag@CP-Ni-NDC after four cycles.
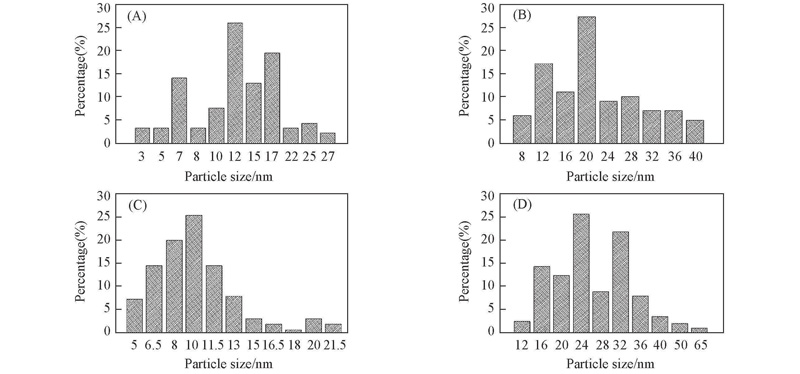
Fig.7 Au particle size distributions of different samples(A) Fresh 4.49%Au@CP-Ni-NDC; (B) 4.49%Au@CP-Ni-NDC after four cycles; (C) fresh 3.43%Ag@CP-Ni-NDC; (D) 3.43%Ag@CP-Ni-NDC after four cycles.
| Entry | Catalyst | Aldehyde | Alkyne | t/h | Yield(%) | TONb |
|---|---|---|---|---|---|---|
| 1 | | Benzaldehyde | Phenylacetylene | 10 | 2 | |
| 2 | CP-Ni-NDC | Benzaldehyde | Phenylacetylene | 10 | 4 | |
| 3 | 0.81%Au@CP-Ni-NDC | Benzaldehyde | Phenylacetylene | 1 | 9 | 7.7 |
| 4 | 2.03%Au@CP--Ni-NDC | Benzaldehyde | Phenylacetylene | 1 | 22 | 7.5 |
| 5 | 4.49%Au@CP-Ni-NDC | Benzaldehyde | Phenylacetylene | 1 | 50 | 7.8 |
| 6 | 4.49%Au@CP-Ni-NDC-Py | Benzaldehyde | Phenylacetylene | 1 | 35 | 5.5 |
| 7 | 4.49%Au@CP-Ni-NDC | Benzaldehyde | Phenylacetylene | 10 | 63 | 9.9 |
| 8 | 3.43%Ag@CP-Ni-NDC | Benzaldehyde | Phenylacetylene | 1 | 28 | 3.1 |
| 9 | 3.43%Ag@CP-Ni-NDC | Benzaldehyde | Phenylacetylene | 10 | 43 | 4.8 |
Table 1 Coupling of benzaldehyde, phenylacetylene and piperidine catalyzed by different catalystsa
| Entry | Catalyst | Aldehyde | Alkyne | t/h | Yield(%) | TONb |
|---|---|---|---|---|---|---|
| 1 | | Benzaldehyde | Phenylacetylene | 10 | 2 | |
| 2 | CP-Ni-NDC | Benzaldehyde | Phenylacetylene | 10 | 4 | |
| 3 | 0.81%Au@CP-Ni-NDC | Benzaldehyde | Phenylacetylene | 1 | 9 | 7.7 |
| 4 | 2.03%Au@CP--Ni-NDC | Benzaldehyde | Phenylacetylene | 1 | 22 | 7.5 |
| 5 | 4.49%Au@CP-Ni-NDC | Benzaldehyde | Phenylacetylene | 1 | 50 | 7.8 |
| 6 | 4.49%Au@CP-Ni-NDC-Py | Benzaldehyde | Phenylacetylene | 1 | 35 | 5.5 |
| 7 | 4.49%Au@CP-Ni-NDC | Benzaldehyde | Phenylacetylene | 10 | 63 | 9.9 |
| 8 | 3.43%Ag@CP-Ni-NDC | Benzaldehyde | Phenylacetylene | 1 | 28 | 3.1 |
| 9 | 3.43%Ag@CP-Ni-NDC | Benzaldehyde | Phenylacetylene | 10 | 43 | 4.8 |
| Entry | Catalyst | Aldehyde | Alkyne | t/h | Yield(%) | TONb |
|---|---|---|---|---|---|---|
| 1 | 4.49%Au@CP-Ni-NDC | 4-Chlorobenzaldehyde | Phenylacetylene | 10 | 44 | 6.9 |
| 2 | 4-Methylbenzaldehyde | Phenylacetylene | 10 | 97 | 15.2 | |
| 3 | 4-Methoxybenzaldehyde | Phenylacetylene | 10 | 80 | 12.5 | |
| 4 | Cyclohexylaldehyde | Phenylacetylene | 10 | 93 | 14.6 | |
| 5 | n-Octanaldehyde | Phenylacetylene | 10 | 99 | 15.5 | |
| 6 | n-Heptaldehyde | Phenylacetylene | 10 | 99 | 15.5 | |
| 7 | Benzaldehyde | 4-Ethylphenylacetylene | 10 | 36 | 5.6 | |
| 8 | Benzaldehyde | 4-Butylphenylacetylene | 10 | 32 | 5.0 | |
| 9 | Benzaldehyde | 1-Octyne | 10 | 22 | 3.4 | |
| 10 | 3.43%Ag@CP-Ni-NDC | 4-Chlorobenzaldehyde | Phenylacetylene | 10 | 31 | 3.5 |
| 11 | 4-Methylbenzaldehyde | Phenylacetylene | 10 | 75 | 8.4 | |
| 12 | 4-Methoxybenzaldehyde | Phenylacetylene | 10 | 71 | 8.0 | |
| 13 | Cyclohexylaldehyde | Phenylacetylene | 10 | 70 | 7.9 | |
| 14 | n-Octanaldehyde | Phenylacetylene | 10 | 81 | 9.1 | |
| 15 | n-Heptaldehyde | Phenylacetylene | 10 | 99 | 11.1 | |
| 16 | Benzaldehyde | 4-Ethylphenylacetylene | 10 | 27 | 3.0 | |
| 17 | Benzaldehyde | 4-Butylphenylacetylene | 10 | 32 | 3.6 | |
| 18 | Benzaldehyde | 1-Octyne | 10 | 19 | 2.1 |
Table 2 A3 coupling reactions of different aldehydes and alkynes catalyzed by 4.49%Au@CP-Ni-NDC and 3.43%Ag@CP-Ni-NDCa
| Entry | Catalyst | Aldehyde | Alkyne | t/h | Yield(%) | TONb |
|---|---|---|---|---|---|---|
| 1 | 4.49%Au@CP-Ni-NDC | 4-Chlorobenzaldehyde | Phenylacetylene | 10 | 44 | 6.9 |
| 2 | 4-Methylbenzaldehyde | Phenylacetylene | 10 | 97 | 15.2 | |
| 3 | 4-Methoxybenzaldehyde | Phenylacetylene | 10 | 80 | 12.5 | |
| 4 | Cyclohexylaldehyde | Phenylacetylene | 10 | 93 | 14.6 | |
| 5 | n-Octanaldehyde | Phenylacetylene | 10 | 99 | 15.5 | |
| 6 | n-Heptaldehyde | Phenylacetylene | 10 | 99 | 15.5 | |
| 7 | Benzaldehyde | 4-Ethylphenylacetylene | 10 | 36 | 5.6 | |
| 8 | Benzaldehyde | 4-Butylphenylacetylene | 10 | 32 | 5.0 | |
| 9 | Benzaldehyde | 1-Octyne | 10 | 22 | 3.4 | |
| 10 | 3.43%Ag@CP-Ni-NDC | 4-Chlorobenzaldehyde | Phenylacetylene | 10 | 31 | 3.5 |
| 11 | 4-Methylbenzaldehyde | Phenylacetylene | 10 | 75 | 8.4 | |
| 12 | 4-Methoxybenzaldehyde | Phenylacetylene | 10 | 71 | 8.0 | |
| 13 | Cyclohexylaldehyde | Phenylacetylene | 10 | 70 | 7.9 | |
| 14 | n-Octanaldehyde | Phenylacetylene | 10 | 81 | 9.1 | |
| 15 | n-Heptaldehyde | Phenylacetylene | 10 | 99 | 11.1 | |
| 16 | Benzaldehyde | 4-Ethylphenylacetylene | 10 | 27 | 3.0 | |
| 17 | Benzaldehyde | 4-Butylphenylacetylene | 10 | 32 | 3.6 | |
| 18 | Benzaldehyde | 1-Octyne | 10 | 19 | 2.1 |
| Entry | Catalyst | Yield(%) | Entry | Catalyst | Yield(%) |
|---|---|---|---|---|---|
| Fresh | 4.49%Au@CP-Ni-NDC | 63 | Fresh | 3.43%Ag@CP-Ni-NDC | 43 |
| Run 1 | 4.49%Au@CP-Ni-NDC | 44 | Run 1 | 3.43%Ag@CP-Ni-NDC | 39 |
| Run 2 | 4.49%Au@CP-Ni-NDC | 35 | Run 2 | 3.43%Ag@CP-Ni-NDC | 34 |
| Run 3 | 4.49%Au@CP-Ni-NDC | 30 | Run 3 | 3.43%Ag@CP-Ni-NDC | 30 |
Table 3 Reusability of 4.49%Au@CP-Ni-NDC and 3.43%Ag@CP-Ni-NDC in A3 coupling reaction of benzaldehyde, piperidine and phenylacetylene*
| Entry | Catalyst | Yield(%) | Entry | Catalyst | Yield(%) |
|---|---|---|---|---|---|
| Fresh | 4.49%Au@CP-Ni-NDC | 63 | Fresh | 3.43%Ag@CP-Ni-NDC | 43 |
| Run 1 | 4.49%Au@CP-Ni-NDC | 44 | Run 1 | 3.43%Ag@CP-Ni-NDC | 39 |
| Run 2 | 4.49%Au@CP-Ni-NDC | 35 | Run 2 | 3.43%Ag@CP-Ni-NDC | 34 |
| Run 3 | 4.49%Au@CP-Ni-NDC | 30 | Run 3 | 3.43%Ag@CP-Ni-NDC | 30 |
| [1] | Liu L. L., Zhang X., Gao J. S., Xu C. M., Green Chem., 2012, 14(6), 1710—1720 |
| [2] | Li P., Wang L., Tetrahedron, 2007, 38(40), 5455—5459 |
| [3] | Liu L. L., Tai X. S., Zhou X. J., Xin C. L., Yan Y. M., Scientific Reports, 2017, 7(1), 12709—12717 |
| [4] | Cheng M., Li B.G., Chinese Journal of Synthetic Chemistry, 2012,20(1), 1—6 |
| (成明, 李伯刚.合成化学,2012, 20(1), 1—6) | |
| [5] | Wei C. M., Li Z. G., Li C. J., Synlett., 2004, 35(9), 1472—1483 |
| [6] | Xiao F. P., Chen Y. L., Liu Y., Wang J., Tetrahedron, 2008, 64(12), 2755—2761 |
| [7] | Liu L. L., Tai X. S., Zhang N. N., Meng Q. G., Xin C. L., Reac. Kinet. Mech. Cat., 2016, 119(1), 335—348 |
| [8] | Liu L. L., Tai X. S., Zhou X. J., Liu L. J., Chem. Res. Chinese Universities, 2017, 33(2), 231—238 |
| [9] | Lo V. K., Liu Y., Wong M. K., Che C. M., Org. Lett., 2006, 8(8), 1529—1532 |
| [10] | Wei C., Li Z., Li C. J., Org. Lett., 2003, 5(23), 4473—4475 |
| [11] | Gholinejad M., Saadati F., Shaybanizade S., Pullithadathil B., RSC Adv., 2016, 6(6), 4983—4991 |
| [12] | Gommermann N., Koradin C., Polborn K., Knochel P., Angew. Chem. Int. Ed., 2003, 42(46), 5763—5766 |
| [13] | Chen W. W., Nguyen R. V., Li C. J., Tetrahedron Lett., 2009, 50(24), 2895—2898 |
| [14] | Zani L., Alesi S., Cozzi P. G., Bolm C., J. Org. Chem., 2006, 71(4), 1558—1562 |
| [15] | Li P. H., Wang L., Chin. J. Chem., 2010, 36(50), 1076—1080 |
| [16] | Zhang Y., Li P., Wang M., Wang L., J. Org. Chem., 2009, 74(11), 4364—4367 |
| [17] | Wang X. X., Quan Z. J., Wang X. C., Asian J. Org. Chem., 2015, 4(1), 54—61 |
| [18] | Berrichi A., Bachir R., Benabdallah M., Choukchou-Braham N., Tetrahedron Lett., 2015, 56(11), 1302—1306 |
| [19] | Huang B., Yao X., Li C., Adv. Synth. Catal., 2006, 348(12/13), 1528—1532 |
| [20] | Li W., Zou H. Y., Wang N., Qi Y. J., Journal of Changchun Normal University(Natural Science), 2013, 32(1), 65—70 |
| (李伟, 邹恒野, 王楠, 齐艳娟. 长春师范学院学报(自然科学版), 2013, 32(1), 65—70) | |
| [21] | Liu L. L., Zhang X., Rang S. M., Yang Y., Dai X. P., Gao J. S., Xu C. M., He J., RSC Adv., 2014, 4(25), 13093—13107 |
| [22] | Liu L. L., Tai X. S., Liu M. F., Li Y. F, Feng Y. M., Sun X. R., CIESC Journal, 2015, 66(5), 1738—1747 |
| (刘丽丽, 台夕市, 刘美芳, 李玉峰, 冯一民, 孙晓日.化工学报,2015, 66(5), 1738—1747) | |
| [23] | Yong G. P., Tian D., Tong H. W., Liu S. M., J. Mol. Catal. A: Chem., 2010, 323(1/2), 40—44 |
| [24] | Hareesh K., Joshi R. P., Dahiwale S. S., Bhoraskar V. N., Dhole S. D., Vacuum, 2016, 124, 40—45 |
| [25] | Liu L. L., Tai X. S., Yu G. L., Guo H. M., Meng Q. G., Chem. Res. Chinese Universities, 2016, 32(3), 443—450 |
| [26] | Zhang X., Shi H., Xu B. Q., Catal. Today, 2007, 122(3/4), 330—337 |
| [27] | Liu L. L., Tai X. S., Zhou X. J., Materials, 2017, 10, 99—111 |
| [28] | Kidwai M., Bansal V., Kumar A., Mozumdar S., Green Chem., 2007, 9, 742—745 |
| [29] | Yan W. J., Wang R, Xu Z. Q., Xu J. K., Lin L., Shen Z. Q., Zhou Y. F., J. Mol. Catal. A: Chem., 2006, 255(1/2), 81—85 |
| [30] | Zhang X., Corma A., Angew. Chem. Int. Ed., 2008, 47(23), 4358—4361 |
| [1] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [2] | FAN Jianling, TANG Hao, QIN Fengjuan, XU Wenjing, GU Hongfei, PEI Jiajing, CEHN Wenxing. Nitrogen Doped Ultra-thin Carbon Nanosheet Composited Platinum-ruthenium Single Atom Alloy Catalyst for Promoting Electrochemical Hydrogen Evolution Process [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220366. |
| [3] | LIN Zhi, PENG Zhiming, HE Weiqing, SHEN Shaohua. Single-atom and Cluster Photocatalysis: Competition and Cooperation [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220312. |
| [4] | CHENG Qian, YANG Bolong, WU Wenyi, XIANG Zhonghua. S-doped Fe-N-C as Catalysts for Highly Reactive Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220341. |
| [5] | TENG Zhenyuan, ZHANG Qitao, SU Chenliang. Charge Separation and Surface Reaction Mechanisms for Polymeric Single-atom Photocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220325. |
| [6] | WANG Ruyue, WEI Hehe, HUANG Kai, WU Hui. Freezing Synthesis for Single Atom Materials [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220428. |
| [7] | CHU Yuyi, LAN Chang, LUO Ergui, LIU Changpeng, GE Junjie, XING Wei. Single-atom Cerium Sites Designed for Durable Oxygen Reduction Reaction Catalyst with Weak Fenton Effect [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220294. |
| [8] | YANG Jingyi, LI Qinghe, QIAO Botao. Synergistic Catalysis Between Ir Single Atoms and Nanoparticles for N2O Decomposition [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220388. |
| [9] | LIN Gaoxin, WANG Jiacheng. Progress and Perspective on Molybdenum Disulfide with Single-atom Doping Toward Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220321. |
| [10] | REN Shijie, QIAO Sicong, LIU Chongjing, ZHANG Wenhua, SONG Li. Synchrotron Radiation X-Ray Absorption Spectroscopy Research Progress on Platinum Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220466. |
| [11] | WEI Chunhong, JIANG Qian, WANG Panpan, JIANG Chengfa, LIU Yuefeng. Atomic Scale Investigation of Pt Atoms/clusters Promoted Co-catalyzed Fischer-Tropsch Synthesis [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220074. |
| [12] | ZHANG Xinxin, XU Di, WANG Yanqiu, HONG Xinlin, LIU Guoliang, YANG Hengquan. Effect of Mn Promoter on CuFe-based Catalysts for CO2 Hydrogenation to Higher Alcohols [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220187. |
| [13] | ZHAO Runyao, JI Guipeng, LIU Zhimin. Efficient Electrocatalytic CO2 Reduction over Pyrrole Nitrogen-coordinated Single-atom Copper Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220272. |
| [14] | ZHOU Leilei, CHENG Haiyang, ZHAO Fengyu. Research Progress of CO2 Hydrogenation over Pd-based Heterogeneous Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220279. |
| [15] | SONG Youwei, AN Jiangwei, WANG Zheng, WANG Xuhui, QUAN Yanhong, REN Jun, ZHAO Jinxian. Effects of Ag,Zn,Pd-doping on Catalytic Performance of Copper Catalyst for Selective Hydrogenation of Dimethyl Oxalate [J]. Chem. J. Chinese Universities, 2022, 43(6): 20210842. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||