

Chem. J. Chinese Universities ›› 2020, Vol. 41 ›› Issue (3): 490.doi: 10.7503/cjcu20190317
• Organic Chemistry • Previous Articles Next Articles
TIAN Xia1,YANG Fuqun1,YUAN Wei1,ZHAO Lei1,YAO Lei1,ZHEN Xiaoli1,HAN Jianrong1,*,LIU Shouxin2,*
Received:2019-05-31
Online:2020-02-26
Published:2019-12-10
Contact:
Jianrong HAN,Shouxin LIU
Supported by:CLC Number:
TrendMD:
TIAN Xia,YANG Fuqun,YUAN Wei,ZHAO Lei,YAO Lei,ZHEN Xiaoli,HAN Jianrong,LIU Shouxin. Synthesis, Structure and Recognition Properties of Macrocyclic Crown Ethers with Oxadiazole †[J]. Chem. J. Chinese Universities, 2020, 41(3): 490.
| Crown ether | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C20H22N2O5 | C18H16N2O4 | C20H20.38N2O5.19 | C22H24N2O6 |
| Formula weight | 370.40 | 324.33 | 371.76 | 412.43 |
| Temperature/K | 293(2) | 113(2) | 113(2) | 113(2) |
| Crystal system | Monoclinic | Orthorhombic | Monoclinic | Orthorhombic |
| Space group | P21/n | Pna21 | C2/c | Pbca |
| a/nm | 0.77264(15) | 0.83211(17) | 2.9681(6) | 0.127122(18) |
| b/nm | 1.3886(3) | 1.1948(2) | 0.89442(18) | 0.83364(12) |
| c/nm | 1.6911(3) | 1.5156(3) | 2.9443(6) | 3.7002(5) |
| α/(°) | 90.00 | 90.00 | 90.00 | 90.00 |
| β/(°) | 96.42(3) | 90.00 | 91.61(3) | 90.00 |
| γ/(°) | 90.00 | 90.00 | 90.00 | 90.00 |
| Volume/nm3 | 0.784 | 1.506.8(5) | 7.813(3) | 3.9212(10) |
| Z | 4 | 4 | 16 | 8 |
| F(000) | 408 | 680 | 3134 | 1744 |
| Crystal size/mm-3 | 0.16×0.14×0.10 | 0.18×0.16×0.14 | 0.20×0.18×0.12 | 0.22×0.20×0.1 |
| 2θ range/(°) | 2.42—27.87 | 2.17—27.51 | 2.48—25.02 | 1.10—27.88 |
| Index range | -9≤h≤10, -13≤k≤18, -20≤l≤22 | -10≤h≤10, -15≤k≤15, -19≤l≤19 | -35≤h≤24, -10≤k≤10, -34≤l≤35 | -6≤h≤9, -16≤k≤16, -19≤l≤20 |
| Data/restraint/parameter | 4290/0/246 | 3450/1/217 | 6832/317/566 | 4665/0/271 |
| Final R indices [I >2σ(I)], wR2(all data) | R1=0.0403, wR2=0.1043 | R1=0.0387, wR2=0.0933 | R1=0.0825, wR2=0.2257 | R1=0.0415, wR2=0.1042 |
| Crown ether | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C20H22N2O5 | C18H16N2O4 | C20H20.38N2O5.19 | C22H24N2O6 |
| Formula weight | 370.40 | 324.33 | 371.76 | 412.43 |
| Temperature/K | 293(2) | 113(2) | 113(2) | 113(2) |
| Crystal system | Monoclinic | Orthorhombic | Monoclinic | Orthorhombic |
| Space group | P21/n | Pna21 | C2/c | Pbca |
| a/nm | 0.77264(15) | 0.83211(17) | 2.9681(6) | 0.127122(18) |
| b/nm | 1.3886(3) | 1.1948(2) | 0.89442(18) | 0.83364(12) |
| c/nm | 1.6911(3) | 1.5156(3) | 2.9443(6) | 3.7002(5) |
| α/(°) | 90.00 | 90.00 | 90.00 | 90.00 |
| β/(°) | 96.42(3) | 90.00 | 91.61(3) | 90.00 |
| γ/(°) | 90.00 | 90.00 | 90.00 | 90.00 |
| Volume/nm3 | 0.784 | 1.506.8(5) | 7.813(3) | 3.9212(10) |
| Z | 4 | 4 | 16 | 8 |
| F(000) | 408 | 680 | 3134 | 1744 |
| Crystal size/mm-3 | 0.16×0.14×0.10 | 0.18×0.16×0.14 | 0.20×0.18×0.12 | 0.22×0.20×0.1 |
| 2θ range/(°) | 2.42—27.87 | 2.17—27.51 | 2.48—25.02 | 1.10—27.88 |
| Index range | -9≤h≤10, -13≤k≤18, -20≤l≤22 | -10≤h≤10, -15≤k≤15, -19≤l≤19 | -35≤h≤24, -10≤k≤10, -34≤l≤35 | -6≤h≤9, -16≤k≤16, -19≤l≤20 |
| Data/restraint/parameter | 4290/0/246 | 3450/1/217 | 6832/317/566 | 4665/0/271 |
| Final R indices [I >2σ(I)], wR2(all data) | R1=0.0403, wR2=0.1043 | R1=0.0387, wR2=0.0933 | R1=0.0825, wR2=0.2257 | R1=0.0415, wR2=0.1042 |
| Crown ether | D—H…A | d(D—H) | d(H A) | d(D…A) | ∠DHA |
|---|---|---|---|---|---|
| 1 | C5—H5…N2a | 0.093 | 0.262 | 0.33846(17) | 140 |
| C20—H20B…O1b | 0.096 | 0.251 | 0.33451(17) | 146 | |
| 2 | C11—H11A…O3c | 0.097 | 0.247 | 0.3409(2) | 163 |
| C11—H11A…O3d | 0.097 | 0.259 | 0.2904(2) | 100 | |
| 3 | C35—H35A…N4e | 0.093 | 0.248 | 0.3398(3) | 169 |
| 4 | C14—H14b…O4f | 0.099 | 0.258 | 0.35623(17) | 171 |
| C15—H15B…N2 g | 0.099 | 0.262 | 0.31511(18) | 114 |
| Crown ether | D—H…A | d(D—H) | d(H A) | d(D…A) | ∠DHA |
|---|---|---|---|---|---|
| 1 | C5—H5…N2a | 0.093 | 0.262 | 0.33846(17) | 140 |
| C20—H20B…O1b | 0.096 | 0.251 | 0.33451(17) | 146 | |
| 2 | C11—H11A…O3c | 0.097 | 0.247 | 0.3409(2) | 163 |
| C11—H11A…O3d | 0.097 | 0.259 | 0.2904(2) | 100 | |
| 3 | C35—H35A…N4e | 0.093 | 0.248 | 0.3398(3) | 169 |
| 4 | C14—H14b…O4f | 0.099 | 0.258 | 0.35623(17) | 171 |
| C15—H15B…N2 g | 0.099 | 0.262 | 0.31511(18) | 114 |
| Crown ether | Stability constant(lgKs) | |||||
|---|---|---|---|---|---|---|
| Li+ | Na+ | K+ | Rb+ | Mg2+ | Ca2+ | |
| 1 | 6.58 | 5.72 | 5.30 | 14.69 | ||
| 2 | 6.72 | 6.81 | 6.35 | |||
| 3 | 4.44 | 5.37 | 5.85 | 5.26 | ||
| 4 | 6.45 | 6.49 | 1.83 | 5.20 | ||
| Crown ether | Stability constant(lgKs) | |||||
|---|---|---|---|---|---|---|
| Li+ | Na+ | K+ | Rb+ | Mg2+ | Ca2+ | |
| 1 | 6.58 | 5.72 | 5.30 | 14.69 | ||
| 2 | 6.72 | 6.81 | 6.35 | |||
| 3 | 4.44 | 5.37 | 5.85 | 5.26 | ||
| 4 | 6.45 | 6.49 | 1.83 | 5.20 | ||
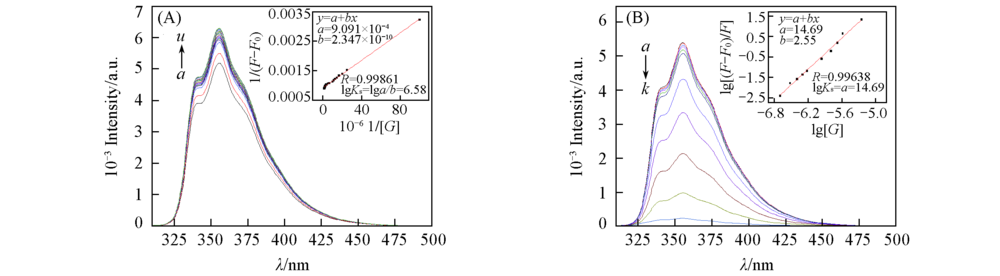
Fig.3 Fluorescence spectra of crown ether 1(1.0×10-6 mol/L) in the presence of differeat concentrations of sodium perchlorate(A) and calcium perchlorate(B) in CH3CN (A) 106c(sodium perchlorate)/(mol·L-1), a—u: 0, 0.1, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.5, 2.0, 2.5, 3.0, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5. Inset: the linear fit plot of the 1/(F-F0) as a function of 1/[G] to calculate the complex stability constants. (B) 106c(calcium perchlorate)/(mol·L-1), a—k: 0, 0.2, 0.3, 0.4, 0.5, 0.6, 1.1, 1.6, 2.1, 2.6, 5.6. Inset: the linear fit plot of lg[(F0-F)/F] as a function of lg[G] to calculate the complex stability constants. λex=300 nm.
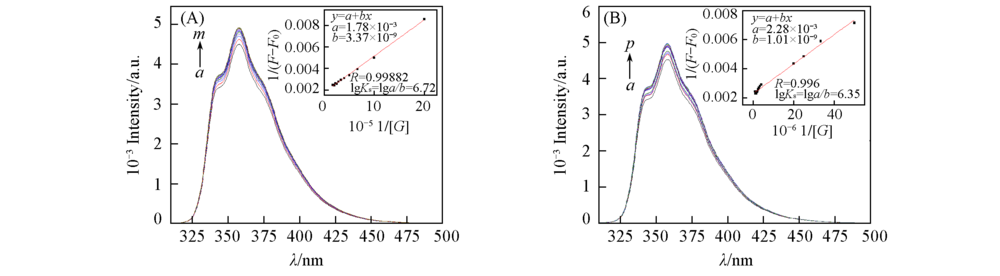
Fig.4 Fluorescence spectra of crown ether 2(1.0×10-6 mol/L) in the presence of different concentrations of sodium perchlorate(A) and calcium perchlorate(B ) in CH3CN (A) 106c(sodium perchlorate)/(mol·L-1), a—m: 0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0; (B) 106c(calcium perchlorate)/(mol·L-1), a—p: 0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 9.0. Insets: the linear fit plot of the 1/(F-F0) as a function of 1/[G] to calculate the complex stability constants. λex=300 nm.
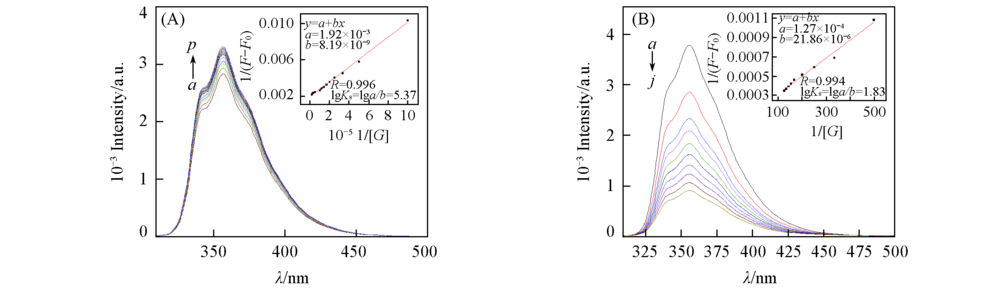
Fig.5 Fluorescence spectra of crown ether 3(1.0×10-6 mol/L) in the presence of different concentrations of sodium perchlorate in CH3CN(A) and fluorescence spectra of crown ether 4(5.0×10-7 mol/L) in the presence of different concentrations of magnesium perchlorate in CH3CN(B) (A) 106c(sodium perchlorate)/(mol·L-1), a—p: 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 20, 30, 40, 50, 60. (B) 103c(magnesium perchlorate)/(mol·L-1), a—j: 0, 2.0, 3.0, 4.0, 5.0, 6.0,6.5,7.0, 8.0. Inset: the linear fit plot of the 1/(F-F0) as a function of 1/[G] to calculate the complex stability constants. λex=300 nm.
| [1] | Zhang C. L., Gong R. Q., Yang J. Y., Sun X. N., Li Y. L., Wang H. Y., Song F. L., Sun Y. D ., Chem. J. Chinese Universities, 2019, 40( 2), 262— 271 |
| ( 张成路, 宫荣庆, 杨敬怡, 孙晓娜, 李奕嶙, 王华玉, 宋府璐, 孙越冬 . 高等学校化学学报, 2019, 40( 2), 262— 271) | |
| [2] | Ilango K., Valentina P., Kumar G., Dixit D., Nilewar S., Kathiravan M. K ., Med. Chem., 2015, 11, 753— 763 |
| [3] | Verma G., Chashoo G., Ali A., Khan M. F., Akhtar W., Ali I., Akhtar M., Alam M. M., Shaquiquzzaman M ., Bioorg. Chem., 2018, 77, 106— 124 |
| [4] | Bostrom J., Hogner A., Llinas A., Wellner E., Plowright A. T ., J. Med. Chem., 2012, 55, 1817— 1830 |
| [5] | Naresh K. R., Poornachandra Y., Nagender P., Santhosh K. G., Krishna S. D., Ganesh K. C., Narsaiah B ., Bioorg. Med. Chem. Lett., 2016, 26, 4829— 4831 |
| [6] | Hamciuc C., Hamciuc E., Homocianu M., Nicolescu A., Carja I. D ., Dyes and Pigments, 2015, 114, 110— 123 |
| [7] | Wu X. M., Wang L., Hua Y. L., Wang C. S., Andrei S. B., Martin R. B ., Tetrahedron, 2014, 70( 11), 2015— 2019 |
| [8] | Paraschivescu C. C., Hǎdade N. D., Coman A. G., Gautier A., Cisnetti F., Matache M ., Tetrahedron Lett., 2015, 56( 25), 3961— 3964 |
| [9] | Wang C. S., Tung G. Y., Hua Y. L ., Chem. Mater., 2001, 13, 1167— 1173 |
| [10] | Zhang Y. D., Jespersen K. G., Kempe M ., Langmuir, 2003, 19, 6534— 6536 |
| [11] | Pradipta P., Nitin C ., J. Mol. Struct., 2001, 570, 145— 152 |
| [12] | Du D. M., Hua W. T., Wang Z. M., Yan C.H ., Heteroat. Chem., 2001, 12( 6), 480— 484 |
| [13] | Du D. M., Hua W. T ., Synth. Commun., 2001, 31( 20), 3197— 3203 |
| [14] | Zhou J. M., Hua W. T., Yang Q. C ., Chem. J. Chinese Universities, 1996, 17( 11), 1721— 1724 |
| ( 周健民, 花文廷, 杨清传 . 高等学校化学学报, 1996, 17( 11), 1721— 1724) | |
| [15] | Tian X., Zhen X. L., Han J. R., Ming C. X., Liu S. X ., Acta Crystallogr. Sect. E: Struct. Rep., 2009, 65, 2010 |
| [16] | Liu Y., Duan Z. Y., Zhang H. Y., Jiang X. L., Han J. R ., J. Org. Chem., 2005, 70, 1450— 1455 |
| [17] | Zhang B. W., Li B., Xia W. S., Ding L ., Prog. Pharm. Sci., 2011, 35( 7), 297— 303 |
| ( 张冰卫, 李博, 夏文水, 丁黎 . 药学进展, 2011, 35( 7), 297— 303) |
| [1] | LIU Qingqing, WANG Pu, WANG Yongshuai, ZHAO Man, DONG Huanli. Synthesis and Topochemical Polymerization Study of Naphthalene/perylene Imides Substituted Diacetylene Derivatives [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220091. |
| [2] | SHI Naike, ZHANG Ya, SANSON Andrea, WANG Lei, CHEN Jun. Uniaxial Negative Thermal Expansion and Mechanism in Zn(NCN) [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220124. |
| [3] | CAO Lei, CHEN Meijun, YUAN Gang, CHANG Gang, ZHANG Xiuhua, WANG Shengfu, HE Hanping. Solution-gated Graphene Field Effect Transistor Sensor Based on Crown Ether Functionalization for the Detection of Mercury Ion [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210688. |
| [4] | YUE Shengli, WU Guangbao, LI Xing, LI Kang, HUANG Gaosheng, TANG Yi, ZHOU Huiqiong. Research Progress of Quasi-two-dimensional Perovskite Solar Cells [J]. Chem. J. Chinese Universities, 2021, 42(6): 1648. |
| [5] | LIU Dongmei,SU Yajing,LI Shanshan,XU Qiwei,LI Xia. Transition Metal Coordination Polymers Constructed by 4-(4-Carboxyphenoxy)isophthalic Acid: Synthesis, Crystal Structure, Fluorescence Sensing and Photocatalysis † [J]. Chem. J. Chinese Universities, 2020, 41(2): 253. |
| [6] | WANG Mengyu, CAO Simin, LI Haoyang, ZHANG Mengjie, LI Dong, ZHAO Zenan, XU Jianhua. Fluorescence Resonance Energy Transfer Between Coenzyme NADH and Tryptophan [J]. Chem. J. Chinese Universities, 2020, 41(11): 2473. |
| [7] | QIN Liulei,LIU Yang,GUAN Xiaoqin,ZHENG Xiaoyuan,ZHANG Ziyu,LIU Zunqi. Synthesis and Switchable Dielectric Properties of an Inorganic-organic Hybrid Complex [H2(DABCO)CuCl4]·H2O † [J]. Chem. J. Chinese Universities, 2020, 41(1): 70. |
| [8] | CHENG Xiankun, HOU Xue, TIAN Huan, ZHANG Menglong, WEI Hao, ZHAO Zhuo. Synthesis of Macrocyclic Thiacrown Ethers and Their Selective Extraction for Ag(Ⅰ) and Tl(Ⅰ) † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1881. |
| [9] | WEI Xin, DENG Yaoliang, ZHENG Xuming, ZHAO Yanying. Ground Structure and Excited State Proton Transfer Reaction of 2-Aminobenzothiazole [J]. Chem. J. Chinese Universities, 2019, 40(8): 1679. |
| [10] | LI Bing,WANG Xuemin,BAI Fengying,LIU Shuqing. Synthesises, Structures and Antibacterial Activities of a Series of Rare Earth Nitrogen Heterocyclic Complexes† [J]. Chem. J. Chinese Universities, 2019, 40(4): 632. |
| [11] | WANG Dongmei,LIU Zihua,LI Guanghua,LIU Yunling,LI Chunxia. Synthesis, Structure and Fluorescent Property of Indium-based Bimetallic Metal-organic Frameworks† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1886. |
| [12] |
TIAN Huan, ZHANG Menglong, WANG Lisha, TONG Bihai, ZHAO Zhuo.
Synthesis of 4,13-Dithio Benzene and-18-Crown-6 and Its Selective Extractability on A |
| [13] | SHI Penghui,BIAN Liujiao. Mechanism Study on the Interaction Between Cefoxitin and Metal β-Lactamase BcⅡ Based on Spectroscopic Methods and Computional Simulations† [J]. Chem. J. Chinese Universities, 2018, 39(5): 971. |
| [14] | ZHU Lei,HAN Junyan,CHANG Haizhen,QIU Yuyuan,ZHANG Yanan,PENG Danni,HU Wei,MIAO Shaobin. Different Pathways for the Cyclocondensation Reactions of 1,2-Diamine and 1,2-Diketone† [J]. Chem. J. Chinese Universities, 2018, 39(12): 2686. |
| [15] | WANG Yan, CHEN Ping, WANG Yunfei, LIU Guiying, YANG Xi, SU Ying, LI Junyang, LIU Weiwei, LIN Lie. Spectral Characterization of the Interaction Between Methamphetamine and Serum Albumin† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2507. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||