

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (1): 28.doi: 10.7503/cjcu20160514
• Analytical Chemistry • Previous Articles Next Articles
ZHANG Jing1, CHEN Linfeng1, ZHU Yaxian2, ZHANG Yong1,*( )
)
Received:2016-07-18
Online:2017-01-10
Published:2016-12-09
Contact:
ZHANG Yong
E-mail:yzhang@xmu.edu.cn
Supported by:CLC Number:
TrendMD:
ZHANG Jing, CHEN Linfeng, ZHU Yaxian, ZHANG Yong. Effects of Hydroxypropyl-β-cyclodextrin(HPCD) on the Interaction of 1-Hydroxypyrene with Bovine Serum Albumin†[J]. Chem. J. Chinese Universities, 2017, 38(1): 28.
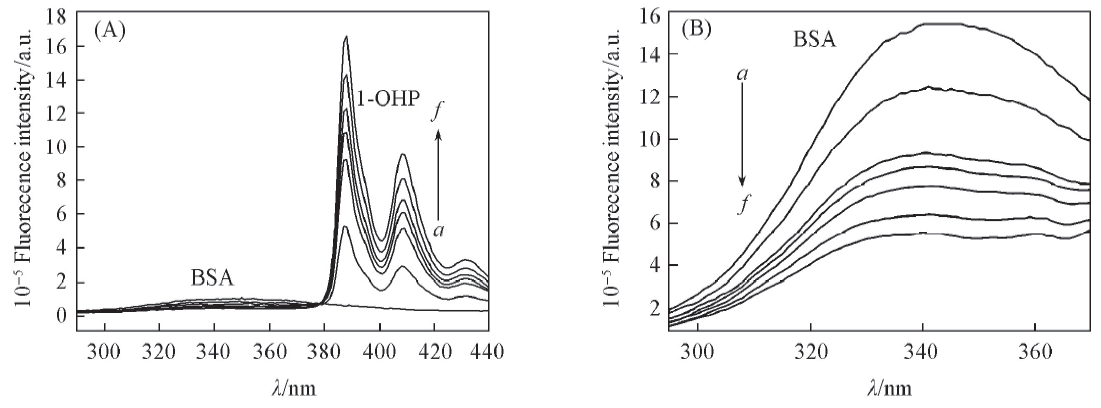
Fig.1 Fluorescence emission spectra of 1-OHPyr-BSA system in the presence of HPCD105 c(1-OHPyr)/(mol·L-1), a—f: 0, 0.2, 0.4, 0.5, 0.6, 0.8, 1.0; c(BSA)=1.0×10-6 mol/L; c(HPCD)=3.0×10-3 mol/L, 291 K. (A) Ex. slit: 1 nm; Em. slit: 1 nm; (B) Ex. slit: 2 nm; Em. slit: 2 nm.
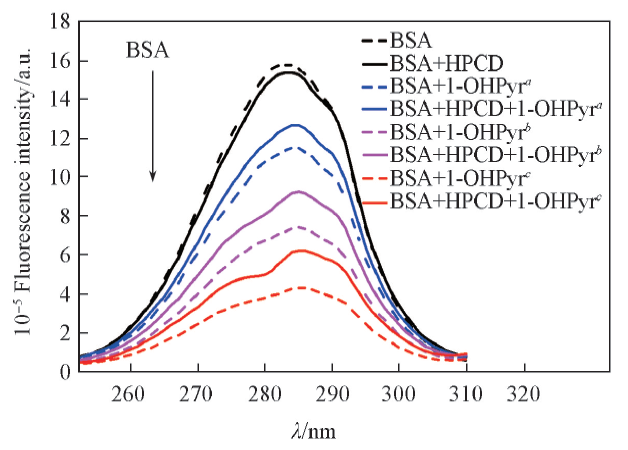
Fig.3 Effects of HPCD on the synchronous fluorescence spectra of 1-OHPyr-BSA systemΔλ=60 nm; c(BSA)=5.0×10-6 mol/L; 105 c(1-OHPyr)/(mol·L-1): a. 0; b. 0.5; c. 1.0. c(HPCD)=3.0×10-3 mol/L.
| c(1-OHPyr)/(mol·L-1) | 1-OHPyr-BSA | 1-OHPyr-BSA-HPCD | ||
|---|---|---|---|---|
| λmax /nm | F/a.u. | λmax /nm | F/a.u. | |
| 0 | 282 | 1.58×106 | 282 | 1.54×106 |
| 1.0×10-6 | 284 | 1.15×106 | 284 | 1.27×106 |
| 5.0×10-6 | 285 | 7.43×105 | 285 | 9.27×106 |
| 1.0×10-5 | 286 | 4.32×105 | 285 | 6.25×106 |
Table 1 Effects of HPCD on the characteristics of BSA synchronous fluorescence peak in 1-OHPyr-BSA system*
| c(1-OHPyr)/(mol·L-1) | 1-OHPyr-BSA | 1-OHPyr-BSA-HPCD | ||
|---|---|---|---|---|
| λmax /nm | F/a.u. | λmax /nm | F/a.u. | |
| 0 | 282 | 1.58×106 | 282 | 1.54×106 |
| 1.0×10-6 | 284 | 1.15×106 | 284 | 1.27×106 |
| 5.0×10-6 | 285 | 7.43×105 | 285 | 9.27×106 |
| 1.0×10-5 | 286 | 4.32×105 | 285 | 6.25×106 |
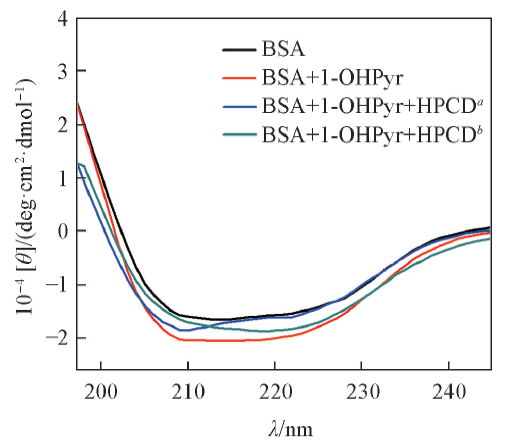
Fig.4 CD spectra of the 1-OHPyr-BSA systems with different concentrations of HPCDc(BSA)=4.0×10-8 mol/L; c(1-OHPyr)=4.0×10-7 mol/L; c(HPCD): a. 3.0×10-5 mol/L; b. 3.0×10-4 mol/L.
| System | α-Helix(%) | β-Sheet(%) | Turn(%) | Random coil(%) | NRMSDa |
|---|---|---|---|---|---|
| BSA | 50.9 | 11.6 | 13.1 | 24.5 | 0.101 |
| 1-OHPyr-BSA | 54.5 | 8.2 | 14.6 | 22.7 | 0.083 |
| 1-OHPyr-BSA-HPCDb | 53.6 | 11.0 | 8.9 | 26.6 | 0.471 |
| 1-OHPyr-BSA-HPCDc | 51.8 | 10.2 | 12.0 | 26.1 | 0.094 |
Table 2 Secondary structural contents of BSA in different systems
| System | α-Helix(%) | β-Sheet(%) | Turn(%) | Random coil(%) | NRMSDa |
|---|---|---|---|---|---|
| BSA | 50.9 | 11.6 | 13.1 | 24.5 | 0.101 |
| 1-OHPyr-BSA | 54.5 | 8.2 | 14.6 | 22.7 | 0.083 |
| 1-OHPyr-BSA-HPCDb | 53.6 | 11.0 | 8.9 | 26.6 | 0.471 |
| 1-OHPyr-BSA-HPCDc | 51.8 | 10.2 | 12.0 | 26.1 | 0.094 |
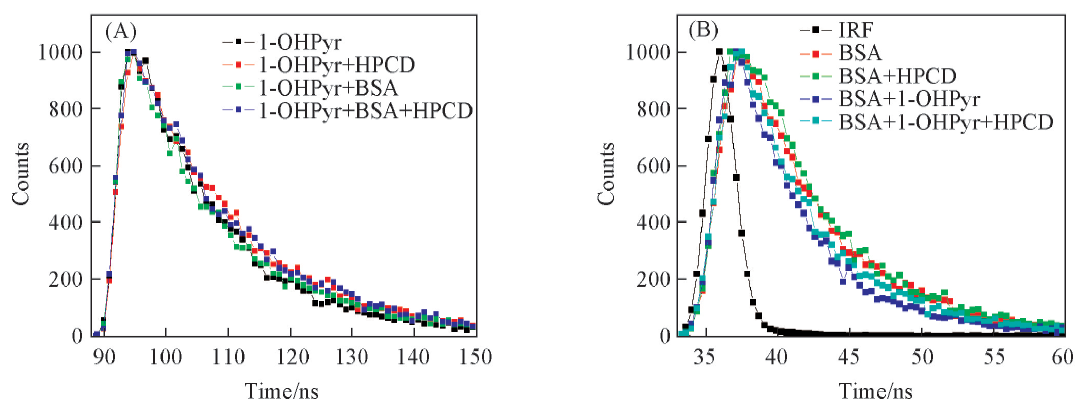
Fig.5 Fluorescence decay curves for 1-OHPyr (A) and BSA (B) in different systems(A) λex=346 nm, λem=386 nm; (B) λex=282 nm, λem=341 nm; c(BSA)=c(1-OHPyr)=5.0×10-6 mol/L; c(HPCD)=3.0×10-3 mol/L.
| System | τf(1-OHPyr)/ns | χ2 | System | τf(BSA)/ns | χ2 |
|---|---|---|---|---|---|
| 1-OHPyr | 14.73 | 1.066 | BSA | 5.98 | 1.051 |
| 1-OHPyr-HPCD | 18.04 | 1.069 | BSA-HPCD | 6.06 | 1.157 |
| 1-OHPyr-BSA | 16.21 | 1.070 | 1-OHPyr-BSA | 4.59 | 1.024 |
| 1-OHPyr-BSA-HPCD | 17.01 | 0.997 | 1-OHPyr-BSA-HPCD | 5.07 | 1.117 |
Table 3 Fluorescence decay parameters for BSA and 1-OHPyr in different systems at 291 K*
| System | τf(1-OHPyr)/ns | χ2 | System | τf(BSA)/ns | χ2 |
|---|---|---|---|---|---|
| 1-OHPyr | 14.73 | 1.066 | BSA | 5.98 | 1.051 |
| 1-OHPyr-HPCD | 18.04 | 1.069 | BSA-HPCD | 6.06 | 1.157 |
| 1-OHPyr-BSA | 16.21 | 1.070 | 1-OHPyr-BSA | 4.59 | 1.024 |
| 1-OHPyr-BSA-HPCD | 17.01 | 0.997 | 1-OHPyr-BSA-HPCD | 5.07 | 1.117 |
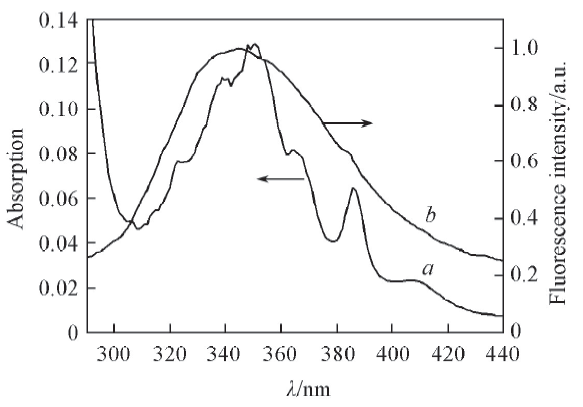
Fig.6 Overlap of UV-Vis absorption spectrum of 1-OHPyr(a) in the presence of HPCD with the fluorescence emission spectrum of BSA(b)c(BSA)=c(1-OHPyr)=5.0×10-6 mol/L; c(HPCD)=3.0×10-3 mol/L.
| System | J/(cm3·L·mol-1) | E(%) | R0/nm | r/nm |
|---|---|---|---|---|
| 1-OHPyr-BSA[ | 1.37×10-14 | 40 | 2.69 | 2.88 |
| 1-OHPyr-BSA-HPCD | 2.16×10-14 | 21 | 2.85 | 3.55 |
Table 4 Energy transfer parameters between 1-OHPyr and BSA in the absence and presence of HPCD*
| System | J/(cm3·L·mol-1) | E(%) | R0/nm | r/nm |
|---|---|---|---|---|
| 1-OHPyr-BSA[ | 1.37×10-14 | 40 | 2.69 | 2.88 |
| 1-OHPyr-BSA-HPCD | 2.16×10-14 | 21 | 2.85 | 3.55 |
| [1] | Elsadek B., Kratz F., J. Control. Release,2012, 157(1), 4—28 |
| [2] | Boström C. E., Gerde P., Hanberg A., Jernström B., Johansson C., Kyrklund T., Rannug A., Törnqvist M., Victorin K., Westerholm R., Environ. Health Persp., 2002, 110(Suppl 3), 451—488 |
| [3] | Kato Y., Onishi M., Haraguchi K., Ikushiro S., Ohta C., Koga N., Endo T., Yamada S., Degawa M., Toxicol. Appl. Pharm., 2011, 254(1), 48—55 |
| [4] | Liu S. F., Wang H. L., Song M. Y., Yin J. F., Jiang G. B., Electrophoresis,2008, 29(14), 3038—3046 |
| [5] | Xu C. B., Gu J. L., Ma X. P., Dong T., Meng X. L., Spectrochim. Acta A,2014, 125, 391—395 |
| [6] | Zhang J., Chen W., Tang B., Zhang W., Chen L., Duan Y., Zhu Y., Zhu Y., Zhang Y., RSC Adv., 2016, 6(28), 23622—23633 |
| [7] | Couthon F., Clottes E., Vial C., Biochem. Bioph. Res. Co., 1996, 227(3), 854—860 |
| [8] | Sudha N., Sameena Y., Enoch I. V., J. Solution Chem., 2016, 45(3), 431—444 |
| [9] | Bolattin M. B., Nandibewoor S. T., Joshi S. D., Dixit S. R., Chimatadar S. A., Ind. Eng. Chem. Res., 2016, 55(19), 5454—5464 |
| [10] | Natesan S., Sowrirajan C., Dhanaraj P., Enoch I. V., B. Chem. Soc., 2014, 35(7), 2114—2122 |
| [11] | Belica S., Navarro-González I., Meseguer J. M. V., Pérez-Sánchez H., Food Chem., 2015, 168, 276—287 |
| [12] | Yan J., Wu D., Ma X. L., Wang L. L., Xu K. L., Li H., Carbohyd. Polym., 2015, 131, 65—74 |
| [13] | Li R., Wu Z., Wang Y., Ding L., Wang Y., J. Biotechnol., 2016, 220(20), 33—34 |
| [14] | Jongeneelen F. J., Ann. Occup. Hyg., 2001, 45(1), 3—13 |
| [15] | Su R. N., Cheong H. K., Ha M., Eom S. Y., Kim H., Choi Y. H., Paek D., Sci. Total Environ., 2015, 515/516, 207—214 |
| [16] | Zhang J., Chen W. X., Zhang W., Duan Y., Zhu Y. X., Zhu Y. X., Zhang Y., Chem. J. Chinese Universities,2015, 36(8), 1511—1516 |
| (张静, 陈薇晓, 张唯, 段滢, 朱玉秀, 朱亚先, 张勇. 高等学校化学学报, 2015, 36(8), 1511—1516) | |
| [17] | Del Valle E. M. M., Process Biochem., 2004, 39(9), 1033—1046 |
| [18] | Peng X., Wang X., Qi W., Su R., He Z., Food Chem., 2016, 192, 178—187 |
| [19] | Lakowicz J.R., Principles of Fluorescence Spectroscopy, 3rd Edition, Springer US, New York, 2006, 353—382 |
| [20] | Ghosh K., Rathi S., Arora D., J. Lumin., 2016, 175, 135—140 |
| [21] | Das A., Suresh Kumar G., J. Biomol. Struct. and Dyn., 2016, 34(4), 1—14 |
| [22] | Li X. D., Fu Y., Ma L. N., Wang Z. X., Zhang H. M., Chem. Res. Chinese Universities,2016, 32(3), 343—347 |
| [23] | Chen L. F., Zhang J., Zhu Y. X., Zhang Y., Chem. J. Chinese. Universities,2015, 36(12), 2394—2401 |
| (陈霖锋, 张静, 朱亚先, 张勇. 高等学校化学学报, 2015, 36(12), 2394—2401) | |
| [24] | Anand U., Mukherjee S., Phys. Chem. Chem. Phys., 2013, 15(23), 9375—9383 |
| [25] | Zhang F., Zhang J., Tong C., Chen Y., Zhuang S., Liu W., J. Hazard. Mater., 2013, 263, 618—626 |
| [26] | Chen L., Zhang J., Zhu Y. X., Zhang Y., RSC Adv., 2015, 5(97), 79874-79881 |
| [27] | Xie X., Lü W., Chen X., J. Hazard. Mater., 2013, 248, 347—354 |
| [28] | Chen M., Liu Y., Cao H., Song L., Zhang Q., J. Lumin., 2015, 158, 116—124 |
| [29] | Ghosh S., Paul B. K., Chattopadhyay N., J. Chem. Sci., 2014, 126(4), 931—944 |
| [30] | Nelson G., Patonay G., Warner I. M., Appl. Spectrosc., 1987, 41(7), 1235—1238 |
| [31] | Wang J., Wang J. X., Xu C., Liu R. T., Chen Y. D., J. Hazard. Mater., 2016, 307, 173—183 |
| [32] | Zhuang S., Wang H., Ding K., Wang J., Pan L., Lu Y., Liu Q., Zhang C., Chemosphere,2016, 144, 1050—1059 |
| [33] | Yorozu T., Hoshino M., Imamura M., J. Phys. Chem., 1982, 86(22), 4426—4429 |
| [34] | Förster T., Delocalized Excitation and Excitation Transfer, in: Sinanoglu O.(Ed.), Modern Quantum Chemistry, Part Ⅲ, Academic Press, New York, 1965, (3), 93—137 |
| [35] | Hu Y., Da L., Spectrochim. Acta A,2014, 121, 230—237 |
| [36] | Liang F. H., Wang D., Ma P. Y., Wang X. H., Song D. Q., Yu Y., Chem. Res. Chinese Universities,2015, 31(5), 724—729 |
| [37] | Yan J., Wu D., Ma X., Wang L., Xu K., Li H., Carbohyd. Polym., 2015, 131, 65—74 |
| [38] | Wang L., Yan J., Li Y., Xu K., Li S., Tang P., Li H., J. Pharmaceut. Biomed., 2016, 117, 453—463 |
| [39] | Yousuf S., Natesan S., Enoch I.V.,J. Biomol. Struct. Dyn., 2016, 1—14 |
| [40] | Bolattin M., Nandibewoor S., Chimatadar S., J. Mol. Recognit., 2016, 29(7), 308—317 |
| [1] | LI Mengshuo, ZHANG Jing, LIU Dan, ZHU Yaxian, ZHANG Yong. Interactions of Pyrene with Human Serum Albumin and Bovine Serum Albumin: Microenvironmental Polarity Differences at Binding Sites [J]. Chem. J. Chinese Universities, 2021, 42(3): 731. |
| [2] | HAO Yuanyuan, WU Qi, LI Ji, GE Chao, MA Chaoying, QIAN Yong, SU Zhi, LIU Hongke. Novel OsⅡ -arene Complexes Based on Bipyridyl Derivative Ligands: Synthesis, Crystal Structure, Anticancer Activity and Interaction with DNA/BSA† [J]. Chem. J. Chinese Universities, 2018, 39(4): 614. |
| [3] | AN Pengjiao, YU Nannan, SUN Ruisheng, SUI Xiaofang, SONG Yuguang. Characterization of the Interaction Between Esterified TAM Radical and Bovine Serum Albumin [J]. Chem. J. Chinese Universities, 2017, 38(8): 1354. |
| [4] | XU Guoqing, HAO Changchun, HE Jianzhen, ZHANG Lei, SUN Runguang. Effect of Bovine Serum Albumin on the Structure of DSPE Monolayer† [J]. Chem. J. Chinese Universities, 2017, 38(12): 2238. |
| [5] | WANG Huichun, WANG Fachun, LI Baolin. Preparation of Cyclodextrin-based Mesoporous Carbon and Its Catalytic Performance† [J]. Chem. J. Chinese Universities, 2016, 37(11): 2076. |
| [6] | ZHANG Jing, CHEN Weixiao, ZHANG Wei, DUAN Ying, ZHU Yuxiu, ZHU Yaxian, ZHANG Yong. Interaction of 1-Hydroxypyrene with BSA Using Fluorescence Anisotropy and Synchronous Fluorescence Analysis Methods† [J]. Chem. J. Chinese Universities, 2015, 36(8): 1511. |
| [7] | YANG Shuilan, SONG Pan, SHE Wenjie, YANG Tianlin. Mechanism of the Interaction Between a Phosphorus-containing Tripod Ligand Europium(Ⅲ) Complex and Bovine Serum Albumin† [J]. Chem. J. Chinese Universities, 2015, 36(7): 1254. |
| [8] | CHEN Linfeng, ZHANG Jing, ZHU Yaxian, ZHANG Yong. Molecular Interactions of 1-Hydroxypyrene with Catalase Revealed by Spectroscopic Methods Combined with Molecular Docking† [J]. Chem. J. Chinese Universities, 2015, 36(12): 2394. |
| [9] | JIANG Jianhong, LI Xu, XIAO Shengxiong, GU Huiwen, LI Chuanhua, YANG Ping, WEI Deliang, HE Dugui, LI Aitao, LI Xia, YAO Feihong, LI Qiangguo. Interaction of 2-{[4-Amino-5-(3,4,5-trimethoxy-benzyl)-pyrimidin-2-ylimino]-methyl}-6-methoxy-phenol with S. pombe Cells and BSA† [J]. Chem. J. Chinese Universities, 2014, 35(4): 831. |
| [10] | WANG Manyuan, ZHANG Chao, LI Jing, LI Zhaoxia, GONG Muxin. Interaction of Antimalarial Components Combination from Artemisia annua L. with Bovine Serum Albumin† [J]. Chem. J. Chinese Universities, 2014, 35(2): 309. |
| [11] | LIU Xiaoxia, DENG Hao, WANG Yanying, LU Zhiwei, ZENG Xianyin, WANG Xianxiang, ZOU Ping, RAO Hanbing. Thermodynamics Studies on the BSA Adsorption onto Zinc Oxide Surfaces with Different Morphologies† [J]. Chem. J. Chinese Universities, 2014, 35(10): 2156. |
| [12] | LIN Hai-Bin, ZHENG Lin, LIN Yu-Qin, ZHOU Zhao-Hui. Study of Interaction Between Bovine Serum Albumin and Cobalt Complexes of Phenanthroline and Nitrilotriacetate by Fluorescence Spectrometry [J]. Chem. J. Chinese Universities, 2013, 34(8): 1818. |
| [13] | HUANG Ying, WANG Juan, GUO Gai-Ying, TAO Zhu, XUE Sai-Feng, ZHU Qian-Jiang, ZHOU Qing-Di. Interaction of 6-Thioguanine with Cucurbit[7]uril and Bovine Serum Albumin by Spectroscopic Method [J]. Chem. J. Chinese Universities, 2013, 34(2): 375. |
| [14] | YANG Shu-Ping, HAN Li-Jun, PAN Yan, WANG Da-Qi, WANG Nan-Nan, WANG Ting. Synthesis, Characterization, Biological Activity and Interaction with Bovine Serum Albumin of 8- or 6-(3-Chlorobenzoyl)coumarin Derivatives [J]. Chem. J. Chinese Universities, 2013, 34(2): 364. |
| [15] | LV Jian-Quan, HU Qin-Qin, DING Ran, ZHANG Xia, ZHOU Xing-Wang. Synthesis and Fluorescence Property of Novel AgInS2@ZnS Quantum Dots [J]. Chem. J. Chinese Universities, 2013, 34(11): 2478. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||