

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (2): 344.doi: 10.7503/cjcu20130863
• Physical Chemistry • Previous Articles Next Articles
GAO Chenggui1, LONG Zhengwen1,*( ), TAN Xingfeng2, LONG Bo1,2,*(
), TAN Xingfeng2, LONG Bo1,2,*( ), ZHANG Weijun3, LONG Chaoyun1, QIN Shuijie1
), ZHANG Weijun3, LONG Chaoyun1, QIN Shuijie1
Received:2013-09-05
Online:2014-02-10
Published:2013-12-30
Contact:
LONG Zhengwen,LONG Bo
E-mail:sci.zwlong@gzu.edu.cn;longbo@gzmu.edu.cn
Supported by:CLC Number:
TrendMD:
GAO Chenggui, LONG Zhengwen, TAN Xingfeng, LONG Bo, ZHANG Weijun, LONG Chaoyun, QIN Shuijie. Theoretical Investigation on the Reaction Between HRnCCH and X(X=H2O, NH3) in Gas Phase†[J]. Chem. J. Chinese Universities, 2014, 35(2): 344.
| H-Bond type | EHB/(kJ·mol-1) | R(H…X)/nm | R(Y…H)/nm | |
|---|---|---|---|---|
| RC1(H2O…HRnCCH) | 4.6 | 0.2435 | 0.1807 | 1761.99 |
| RC2(H3N…HRnCCH) | 7.5 | 0.2277 | 0.1806 | 1699.26 |
| RC3(HRnCCH…OH2) | 5.1 | 0.2238 | 0.1069 | 3403.46 |
| RC4(HRnCCH…NH3) | 3.7 | 0.2318 | 0.1072 | 3351.37 |
| HCCH…OH2 | 1.4b | 0.2186 b | 0.1067 b | 3392.80 b |
| HCCH…NH3 | 1.7 b | 0.2259 b | 0.1071 b | 3334.88 b |
Table 1 Properties of the studied H-bonded complexesa
| H-Bond type | EHB/(kJ·mol-1) | R(H…X)/nm | R(Y…H)/nm | |
|---|---|---|---|---|
| RC1(H2O…HRnCCH) | 4.6 | 0.2435 | 0.1807 | 1761.99 |
| RC2(H3N…HRnCCH) | 7.5 | 0.2277 | 0.1806 | 1699.26 |
| RC3(HRnCCH…OH2) | 5.1 | 0.2238 | 0.1069 | 3403.46 |
| RC4(HRnCCH…NH3) | 3.7 | 0.2318 | 0.1072 | 3351.37 |
| HCCH…OH2 | 1.4b | 0.2186 b | 0.1067 b | 3392.80 b |
| HCCH…NH3 | 1.7 b | 0.2259 b | 0.1071 b | 3334.88 b |
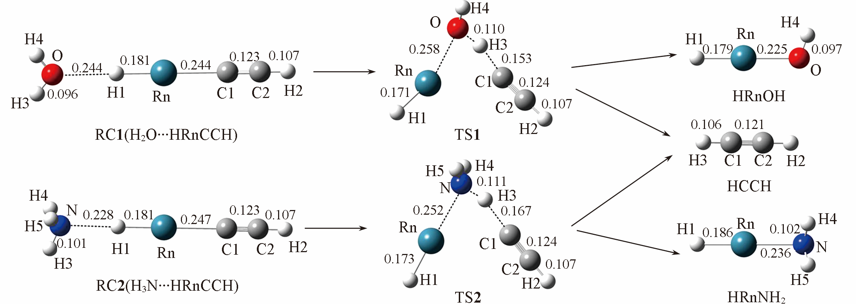
Fig.1 Optimized geometries of H2O…HRnCCH and H3N…HRnCCH and their corresponding transition states and products at MP2/aug-cc-pVTZ-pp level of theory Bond lengths are in nm.
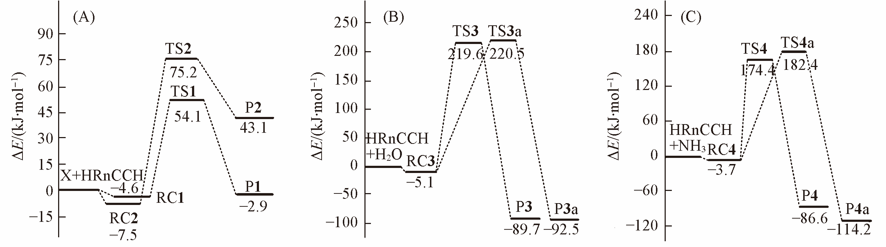
Fig.2 Potential energy profiles at CCSD(T)//MP2/aug-cc-pVTZ-pp level of theoryThe reactions begin with the forming of hydrogen-bonded reactant complexes: (A) X…HRnCCH(X=H2O or NH3);(B) HRnCCH…OH2; (C) HRnCCH…NH3.
| Reaction | ΔHa /(kJ·mol-1) | ΔGa/(kJ·mol-1) | ΔEa/(kJ·mol-1) | ΔEb/(kJ·mol-1) | T1c |
|---|---|---|---|---|---|
| HRnCCH+H2O | 0 | 0 | 0 | 0 | 0.012, 0.010 |
| RC1 | -2.9 | 14.0 | -4.9 | -4.6 | 0.012 |
| TS1 | 42.8 | 79.5 | 47.8 | 54.1 | 0.012 |
| P1 | 22.7 | 29.8 | 27.0 | -2.9 | 0.013, 0.013 |
| RC3 | -5.1 | 15.6 | -75.2 | -5.1 | 0.012 |
| TS3 | 207.9 | 248.3 | 214.3 | 219.6 | 0.015 |
| TS3a | 214.2 | 254.2 | 220.5 | 220.5 | 0.016 |
| P3 | -72.9 | -30.9 | -66.1 | -89.7 | 0.013 |
| P3a | -76.0 | -35.2 | -69.5 | -92.5 | 0.013 |
| HCCH+H2O | 222.2, 241.8, 243.9d | ||||
| HRnCCH+NH3 | 0 | 0 | 0 | 0 | 0.012, 0.008 |
| RC2 | -5.7 | 15.9 | -7.0 | -7.5 | 0.012 |
| TS2 | 67.2 | 102.8 | 71.6 | 75.2 | 0.012 |
| P2 | 23.5 | 23.6 | 25.7 | 43.1 | 0.011, 0.013 |
| RC4 | -7.3 | 14.6 | -8.1 | -3.7 | 0.012 |
| TS4 | 165.9 | 209.6 | 173.2 | 174.4 | 0.014 |
| TS4a | 174.4 | 217.7 | 181.7 | 182.4 | 0.014 |
| P4 | -75.5 | -31.0 | -67.7 | -86.6 | 0.012 |
| P4a | -105.9 | -63.4 | -99.3 | -114.2 | 0.013 |
| HCCH+NH3 | 181.2, 182.0, 190.4, 192.9d | ||||
Table 2 Activated energies(ΔE), enthalpies(ΔH), free energies(ΔG) for the HRnCCH+X(X=H2O, NH3) reactions added with zero-point correction included at 298 K
| Reaction | ΔHa /(kJ·mol-1) | ΔGa/(kJ·mol-1) | ΔEa/(kJ·mol-1) | ΔEb/(kJ·mol-1) | T1c |
|---|---|---|---|---|---|
| HRnCCH+H2O | 0 | 0 | 0 | 0 | 0.012, 0.010 |
| RC1 | -2.9 | 14.0 | -4.9 | -4.6 | 0.012 |
| TS1 | 42.8 | 79.5 | 47.8 | 54.1 | 0.012 |
| P1 | 22.7 | 29.8 | 27.0 | -2.9 | 0.013, 0.013 |
| RC3 | -5.1 | 15.6 | -75.2 | -5.1 | 0.012 |
| TS3 | 207.9 | 248.3 | 214.3 | 219.6 | 0.015 |
| TS3a | 214.2 | 254.2 | 220.5 | 220.5 | 0.016 |
| P3 | -72.9 | -30.9 | -66.1 | -89.7 | 0.013 |
| P3a | -76.0 | -35.2 | -69.5 | -92.5 | 0.013 |
| HCCH+H2O | 222.2, 241.8, 243.9d | ||||
| HRnCCH+NH3 | 0 | 0 | 0 | 0 | 0.012, 0.008 |
| RC2 | -5.7 | 15.9 | -7.0 | -7.5 | 0.012 |
| TS2 | 67.2 | 102.8 | 71.6 | 75.2 | 0.012 |
| P2 | 23.5 | 23.6 | 25.7 | 43.1 | 0.011, 0.013 |
| RC4 | -7.3 | 14.6 | -8.1 | -3.7 | 0.012 |
| TS4 | 165.9 | 209.6 | 173.2 | 174.4 | 0.014 |
| TS4a | 174.4 | 217.7 | 181.7 | 182.4 | 0.014 |
| P4 | -75.5 | -31.0 | -67.7 | -86.6 | 0.012 |
| P4a | -105.9 | -63.4 | -99.3 | -114.2 | 0.013 |
| HCCH+NH3 | 181.2, 182.0, 190.4, 192.9d | ||||
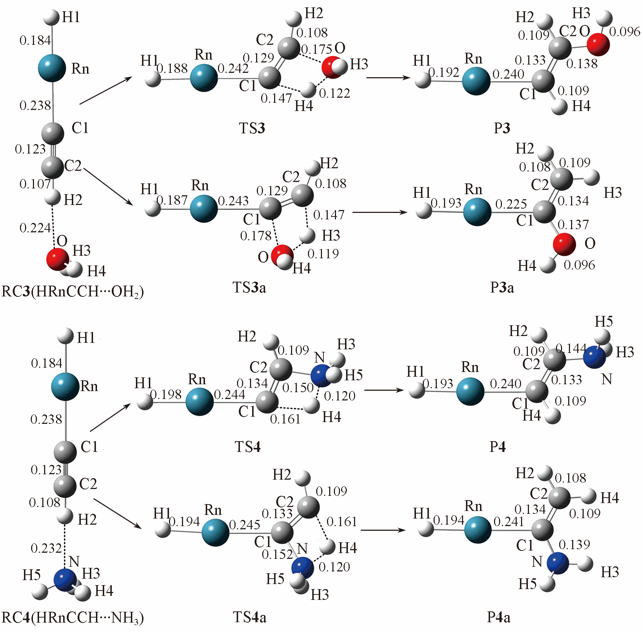
Fig.3 Optimized geometries of HRnCCH…OH2 and HRnCCH…NH3 and their corresponding transition states and products at the MP2/aug-cc-pVTZ-pp level of theoryBond lengths are in nm.
| Reaction | ΔHa /(kJ·mol-1) | ΔGa/(kJ·mol-1) | ΔEa/(kJ·mol-1) | ΔEb/(kJ·mol-1) | T1c |
|---|---|---|---|---|---|
| HRnCCH…H2O+H2O | 0.0 | 0.0 | 0.0 | 0.0 | 0.012, 0.010 |
| R | -45.1 | 16.6 | -41.8 | -55.3 | 0.012 |
| T | 110.8 | 196.4 | 122.9 | 123.2 | 0.016 |
Table 3 Activated energies(ΔE), enthalpies(ΔH) and free energies(ΔG) for the HRnCCH…H2O+H2O reactions with zero-point correction included at 298 K
| Reaction | ΔHa /(kJ·mol-1) | ΔGa/(kJ·mol-1) | ΔEa/(kJ·mol-1) | ΔEb/(kJ·mol-1) | T1c |
|---|---|---|---|---|---|
| HRnCCH…H2O+H2O | 0.0 | 0.0 | 0.0 | 0.0 | 0.012, 0.010 |
| R | -45.1 | 16.6 | -41.8 | -55.3 | 0.012 |
| T | 110.8 | 196.4 | 122.9 | 123.2 | 0.016 |
| [1] | Bartlett N., Proc. Chem. Soc., 1962, 6, 218 |
| [2] | Pettersson M., Lundell J., Räsänen M., J. Chem. Phys., 1995, 102, 6423—6431 |
| [3] | Pettersson M., Lundell J., Räsänen M., J. Chem. Phys., 1995, 103(1), 205—211 |
| [4] | Khriachtchev L., Räsänen M., Gerber R. B., Acc. Chem. Res., 2009, 42(1), 183—191 |
| [5] | Yen S. Y., Mou C. H., Hu W. P., Chem. Phys. Lett., 2003, 383(5/6), 606—611 |
| [6] | Khriachtchev L., Pettersson M., Runeberg N., Lundell J., Räsänen M., Nature, 2000, 406, 874—876 |
| [7] | Pettersson M., Khriachtchev L., Lignell A., Räsänen M., Bihary Z., Gerber R. B., J. Chem. Phys., 2002, 116, 2508—2515 |
| [8] | Pettersson M., Khriachtchev L., Lundell J., Jolkkonen S., Räsänen M., J. Phys. Chem. A, 2000, 104, 3579—3583 |
| [9] | Khriachtchev L., Pettersson M., Lundell J., Räsänen M., J. Chem. Phys., 2001, 114, 7727—7730 |
| [10] | Feldman V. I., Sukhov F. F., Orlov A. Y., Chem. Phys. Lett., 1997, 280, 507—512 |
| [11] | Zhu H., Xie D. Q., Chem. J. Chinese Universities, 2008, 29(1), 174—176 |
| (朱华, 谢代前.高等学校化学学报,2008, 29(1), 174—176) | |
| [12] | Chernick C. L., Claassen H. H., Fields P. R., Hyman H. H., Malm J. G., Manning W. M., Matheson M. S., Quarterman L. A., Schreiner F., Selig H. H., Sheft I., Siegel S., Sloth E. N., Stein L., Studier M. H., Weeks J. L., Zirin M. H., Science, 1962, 138, 136—138 |
| [13] | Stein L., Science, 1970, 168, 362—364 |
| [14] | Stein L., Science, 1972, 175, 1463—1465 |
| [15] | Stein L., J. Inorg. Nucl. Chem., 1973, 35, 39—43 |
| [16] | Stein L., Nature, 1973, 243, 30—32 |
| [17] | Rosalba J., Claudia Z. O., Jimenez-Halla J. O. C., Bickelhaupt F. M., Gabriel M., Phys. Chem. Chem. Phys., 2011, 13, 2222—2227 |
| [18] | Tsivion E., Gerber R. B., Phys. Chem. Chem. Phys., 2010, 12(37), 11791—11794 |
| [19] | Han Y. H., Long Z. W., Long B., Long C. Y., Cai S. H., Zhang W. J., J. At. Mol. Sci., 2012, 3(3), 227—235 |
| [20] | Chang G., Wang B. J., Zhang J., Xia W. S., Wan H. L., Chem. J. Chinese Universities, 2010, 31(9), 1820—1826 |
| (常刚, 王斌举, 张俊, 夏文生, 万惠霖.高等学校化学学报,2010, 31(9), 1820—1826) | |
| [21] | Kryachko E. S., Scheiner S., J. Phys. Chem. A, 2008, 112(9), 1940—1945 |
| [22] | Long B., Chang C. R., Long Z. W., Wang Y. B., Tan X. F., Zhang W. J., Chem. Phys. Lett., 2013, 581, 26—29 |
| [23] | Ren Y., Li M., Wong N. B., J. Mol. Model., 2005, 11(2), 167—173 |
| [24] | Long B., Tan X. F., Ren D. S., Zhang W. J., Chem. Phys. Lett., 2010, 492, 214—219 |
| [25] | Deng C., Wu X.P., Sun X. M., Ren Y., Sheng Y. H., J. Comput. Chem., 2009, 30(2), 285—294 |
| [26] | Long B., Tan X. F., Long Z. W., Wang Y. B., Ren D. S., Zhang W. J., J. Phys. Chem. A, 2011, 115, 6559—6567 |
| [27] | Long B., Tan X. F., Chang C. R., Zhao W. X., Long Z. W., Ren D. S., Zhang W. J., J. Phys. Chem. A, 2013, 117, 5106—5116 |
| [28] | Wei X. G., Sun X. M., Wu X. P., Geng S., Ren Y., Wong N. B., Li W. K., J. Mol. Model., 2011, 17(8), 2069—2082 |
| [29] | Zhu H. J., Ren Y., Ren J., Chu S. Y., J. Mol. Struct.(Theochem.), 2005, 730, 199—205 |
| [30] | Sun X. M., Wei X. G., Wu X. P., Ren Y., Wong N. B., Li W. K., J. Phys. Chem. A, 2010, 114(1), 595—602 |
| [31] | Deng C., Li Q. G., Ren Y., Wong N. B., Chu S. Y., Zhu H. J., J. Comput. Chem., 2008, 29(3), 466—480 |
| [32] | Long B., Zhang W. J., Tan X. F., Long Z. W., Wang Y. B., Ren D. S., J. Phys. Chem. A, 2011, 115, 1350—1357 |
| [33] | Wu X. P., Wei X. G., Sun X. M., Ren Y., Wong N. B., Li W. K., Theor. Chem. Acc., 2010, 127, 493—506 |
| [34] | Chang C. R., Yang X. F., Long B., Li J., ACS. Catal., 2013, 3, 1693—1699 |
| [35] | Long B., Tan X. F., Long Z. W., Ren D. S., Zhang W. J., Chin. J. Chem. Phys., 2011, 24 (1), 16—21 |
| [36] | Gao C. G., Long Z. W., Tan X. F., Long B., Long C. Y., Qin S. J., Zhang W. J., Acta Chim. Sinica, 2013, 71, 849—856 |
| (高成贵, 隆正文, 谭兴凤, 龙波, 龙超云, 秦水介, 张为俊.化学学报, 2013, 71, 849—856) | |
| [37] | Ishida G., Morokuma K., Komornicki A., J. Chem. Phys., 1977, 66(5), 2153—2157 |
| [38] | Xu G.X., Li Y. M., Wang D. M., Quantum Chemistry, 2nd Ed., Science Press, Beijing, 2009, 423—425 |
| (徐光宪, 黎乐民, 王德民.量子化学, 第二版, 北京:科学出版社, 2009, 423—425) | |
| [39] | Rienstra-Kiracofe J. C., Allen W. D., Schaefer H. F., J. Phys. Chem. A, 2000, 104(44), 9823—9840 |
| [40] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Zakrzeski V. G., Montgomery J. J. A., Stratmann R. E., Burant J. C., Dapprich S., Millam J. M., Daniels A. D., Kudin K. N., Strain M. C., Farkas O., Tomasi J., Barone V., Cossi M., Cammi R., Mennucci B., Pomelli C., Adamo C., Clifford S., Ochterski J., Petersson G. A., Ayala P. Y., Cui Q., Morokuma K., Malick D. K., Rabuck A. D., Raghavachari K., Foresman J. B., Cioslowski J., Ortiz J. V., Stefanov B. B., Liu G., Liashenko A., Piskorz P., Komaromi I., Gomperts R., Martin R. L., Fox D. J., Keith T., Al-Laham M. A., Peng C. Y., Nanayakkara A., Gonzalez C., Challacombe M., Gill P. M. W., Johnson B., Chen W., Wong M. W., Andres J. L., Gonzalez C., Head-Gordon M., Replogle E. S., Pople J. A., Gaussian 09, Revision A.02, Gaussian Inc., Wallingford CT, 2009 |
| [1] | TANG Quanjun, LIU Yingxin, MENG Rongwei, ZHANG Ruotian, LING Guowei, ZHANG Chen. Application of Single-atom Catalysis in Marine Energy [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220324. |
| [2] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [3] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [4] | ZHANG Hongwei, CHEN Wen, ZHAO Meiqi, MA Chao, HAN Yunhu. Research Progress of Single Atom Catalysts in Electrochemistry [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220129. |
| [5] | CHEN Changli, MI Wanliang, LI Yujing. Research Progress of Single Atom Catalysts in Electrochemical Hydrogen Cycling [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220065. |
| [6] | LIU Jiaqi, LI Tianbao. Preparation and Photoelectrochemical Performance of BiVO4/CuBi2O4 Thin Film Photoanodes [J]. Chem. J. Chinese Universities, 2022, 43(4): 20220017. |
| [7] | LI Hua, YANG Ke, HUANG Junfeng, CHEN Fengjuan. Design and Construction of UiO-66-NH2/wood Composite for Efficient Removal of Trace Heavy Metal Ions from Water [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210701. |
| [8] | SUN Cuihong, LYU Liqiang, LIU Ying, WANG Yan, YANG Jing, ZHANG Shaowen. Mechanism and Kinetics on the Reaction of Isopropyl Nitrate with Cl, OH and NO3 Radicals [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210591. |
| [9] | TANG Qian, DAN Feijun, GUO Tao, LAN Haichuang. Synthesis and Application of Quinolinone-coumarin-based Colorimetric Fluorescent Probe for Recognition of Hg2+ [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210660. |
| [10] | CHEN Wangsong, LUO Lan, LIU Yuguang, ZHOU Hua, KONG Xianggui, LI Zhenhua, DUAN Haohong. Recent Progress in Photoelectrochemical H2 Production Coupled with Biomass-derived Alcohol/aldehyde Oxidation [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210683. |
| [11] | JIN Keyan, BAI Pu, LI Xiaolong, ZHANG Jianan, YAN Wenfu. New Mg-Al Type Sorbent for Efficient Removal of Boron from Waste Water Containing High-concentration of Boron from Pressurized Water Reactor Nuclear Power Plants [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210516. |
| [12] | LIU Jie, LI Jinsheng, BAI Jingsen, JIN Zhao, GE Junjie, LIU Changpeng, XING Wei. Constructing a Water-blocking Interlayer Containing Sulfonated Carbon Tubes to Reduce Concentration Polarization in Direct Methanol Fuel Cells [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220420. |
| [13] | YANG Zhaohua, CHENG Hongjing, YANG Yi, LIU Hui, DU Feipeng, ZHANG Yunfei. Preparation of Silver-loaded Polyvinyl Alcohol Sponge and Its Interfacial Photothermal Driven Water Evaporation Performance [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220181. |
| [14] | QIAO Zhenghua, FAN Qi, HAO Jingcheng. Silicone Surfactant-enhanced Dual Networks and High Temperature Resistance Porous Silicone Elastomers [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220384. |
| [15] | TIAN Xiaokang, ZHANG Qingsong, YANG Shulin, BAI Jie, CHEN Bingjie, PAN Jie, CHEN Li, WEI Yen. Porous Materials Inspired by Microbial Fermentation: Preparation Method and Application [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220216. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||