

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (7): 1602.doi: 10.7503/cjcu20170698
• Polymer Chemistry • Previous Articles Next Articles
CONG Fei1, SUN Xiuhua1,*( ), WANG Ke2, GUI Taijiang2, GAO Changlu1,*(
), WANG Ke2, GUI Taijiang2, GAO Changlu1,*( )
)
Received:2017-11-06
Online:2018-07-10
Published:2018-06-13
Contact:
SUN Xiuhua,GAO Changlu
E-mail:sunxh@hitwh.edu.cn;clgao@hitwh.edu.cn
Supported by:CLC Number:
TrendMD:
CONG Fei, SUN Xiuhua, WANG Ke, GUI Taijiang, GAO Changlu. Characterization of Tertiary Amine-Cu Complex and Application in Copper Ion Slow-release Coatings†[J]. Chem. J. Chinese Universities, 2018, 39(7): 1602.
| No. | c(Cu)/(mmol·L-1) | c(DMAEMA)/(mmol·L-1) | No. | c(Cu)/(mmol·L-1) | c(DMAEMA)/(mmol·L-1) |
|---|---|---|---|---|---|
| 1 | 1.00 | — | 14 | 1.00 | 5.00 |
| 2 | 2.00 | — | 15 | 1.25 | 5.00 |
| 3 | 3.00 | — | 16 | 1.50 | 5.00 |
| 4 | 4.00 | — | 17 | 1.75 | 5.00 |
| 5 | 5.00 | — | 18 | 2.00 | 5.00 |
| 6 | — | 1.00 | 19 | 2.25 | 5.00 |
| 7 | — | 2.00 | 20 | 2.50 | 5.00 |
| 8 | — | 3.00 | 21 | 2.75 | 5.00 |
| 9 | — | 4.00 | 22 | 3.00 | 5.00 |
| 10 | — | 5.00 | 23 | 3.50 | 5.00 |
| 11 | 0.25 | 5.00 | 24 | 4.00 | 5.00 |
| 12 | 0.50 | 5.00 | 25 | 4.50 | 5.00 |
| 13 | 0.75 | 5.00 | 26 | 5.00 | 5.00 |
Table 1 Composition of the solutions in methanol
| No. | c(Cu)/(mmol·L-1) | c(DMAEMA)/(mmol·L-1) | No. | c(Cu)/(mmol·L-1) | c(DMAEMA)/(mmol·L-1) |
|---|---|---|---|---|---|
| 1 | 1.00 | — | 14 | 1.00 | 5.00 |
| 2 | 2.00 | — | 15 | 1.25 | 5.00 |
| 3 | 3.00 | — | 16 | 1.50 | 5.00 |
| 4 | 4.00 | — | 17 | 1.75 | 5.00 |
| 5 | 5.00 | — | 18 | 2.00 | 5.00 |
| 6 | — | 1.00 | 19 | 2.25 | 5.00 |
| 7 | — | 2.00 | 20 | 2.50 | 5.00 |
| 8 | — | 3.00 | 21 | 2.75 | 5.00 |
| 9 | — | 4.00 | 22 | 3.00 | 5.00 |
| 10 | — | 5.00 | 23 | 3.50 | 5.00 |
| 11 | 0.25 | 5.00 | 24 | 4.00 | 5.00 |
| 12 | 0.50 | 5.00 | 25 | 4.50 | 5.00 |
| 13 | 0.75 | 5.00 | 26 | 5.00 | 5.00 |
| Resin | Molar fraction(%) | Mn | PDI | |||
|---|---|---|---|---|---|---|
| AIBN | DMAEMA | TIPSA | MMA | |||
| PDMAEMA | 1 | 100 | 0 | 0 | 21140 | 2.54 |
| C0 | 1 | — | 30.0 | 70.0 | 24350 | 2.64 |
| C1 | 1 | 10.0 | 10.0 | 80.0 | 22750 | 2.53 |
| C2 | 1 | 12.5 | 7.5 | 80.0 | 21630 | 2.60 |
| C3 | 1 | 15.0 | 5.0 | 80.0 | 22010 | 2.56 |
| C4 | 1 | 17.5 | 2.5 | 80.0 | 21200 | 2.51 |
| C5 | 1 | 20.0 | — | 80.0 | 20100 | 2.43 |
Table 2 Composition of the coatings
| Resin | Molar fraction(%) | Mn | PDI | |||
|---|---|---|---|---|---|---|
| AIBN | DMAEMA | TIPSA | MMA | |||
| PDMAEMA | 1 | 100 | 0 | 0 | 21140 | 2.54 |
| C0 | 1 | — | 30.0 | 70.0 | 24350 | 2.64 |
| C1 | 1 | 10.0 | 10.0 | 80.0 | 22750 | 2.53 |
| C2 | 1 | 12.5 | 7.5 | 80.0 | 21630 | 2.60 |
| C3 | 1 | 15.0 | 5.0 | 80.0 | 22010 | 2.56 |
| C4 | 1 | 17.5 | 2.5 | 80.0 | 21200 | 2.51 |
| C5 | 1 | 20.0 | — | 80.0 | 20100 | 2.43 |
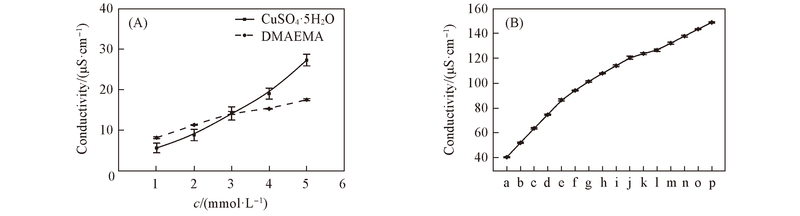
Fig.4 Conductivity of CuSO4·5H2O and DMAEMA in methanol(A) and mixed solutions in methanol(B)^Molar ratio of DMAEMA to CuSO4·5H2O: a. 20∶1; b. 10∶1; c. 6.67∶1; d. 5∶1; e. 4∶1; f. 3.33∶1; g. 2.86∶1; h. 2.5∶1; i. 2.22∶1; j. 2∶1; k. 1.82∶1; l. 1.67∶1; m. 1.43∶1; n. 1.25∶1; o. 1.11∶1; p. 1∶1.
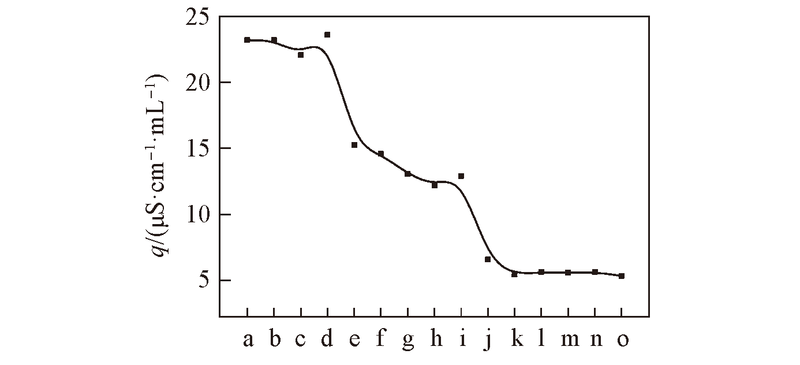
Fig.5 Relation ship between q and molar ratio of DMAEMA to CuSO4·5H2O^Molar ratio of DMAEMA to CuSO4·5H2O: a. 10∶1; b. 6.67∶1; c. 5∶1; d. 4∶1; e. 3.33∶1; f. 2.86∶1; g. 2.5∶1; h. 2.22∶1; i. 2∶1; j. 1.82∶1; k. 1.67∶1; l. 1.43∶1; m. 1.25∶1; n. 1.11∶1; o. 1∶1.
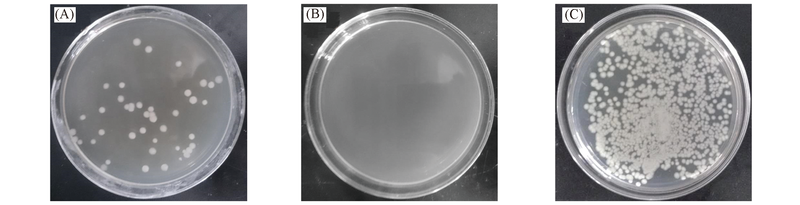
Fig.10 Antibacterial property of the sterile nutrient agar plates prepared with PDMAEMA(A), CuSO4 complex(B) and CuSO4 after releasing in water(C) for 8 d
| [1] | Nurioglu A. G., Esteves A. C. C., de With G., J. Mater. Chem. B, 2015, 3(32), 6547—6570 |
| [2] | Yebra D. M., Kiil S., Dam-Johansen K., Prog. Org. Coat., 2004, 50(2), 75—104 |
| [3] | Chambers L. D., Stokes K. R., Walsh F. C., Wood R. J. K., Surf. Coat. Tech., 2006, 201(6), 3642—3652 |
| [4] | Omae I., Appl. Organomet. Chem., 2003, 17(2), 81—105 |
| [5] | Alzieu C., Ocean Coast. Manage., 1998, 40(1), 23—36 |
| [6] | Lejars M., Margaillan A., Bressy C., Chem. Rev., 2012, 112(8), 4347—4390 |
| [7] | Buskens P., Wouters M., Rentrop C., Vroon Z., J. Coat. Technol. Res. ,2013, 10(1), 29—36 |
| [8] | Ciriminna R., Bright F. V., Pagliaro M., ACS Sustain. Chem. Eng., 2015, 3(4), 559—565 |
| [9] | Li H. J., Wang G. J., Paint & Coatings Industry, 2005, 35(3), 45—49 |
| (李慧娟,王国建.涂料工业, 2005, 35(3), 45—49) | |
| [10] | Yu X. Y., Wang K., Chen Z. T., Yu H. J., Xiao L., Gui T. J., Paint & Coatings Industry, 2012, 2012,42(7), 45—52 |
| (于雪艳, 王科, 陈正涛, 余浩杰, 肖玲, 桂泰江. 涂料工业, 2012,42(7), 45—52) | |
| [11] | Turner A., Mar. Pollut. Bull., 2010, 60(2), 159—171 |
| [12] | Singh N., Turner A., Environ. Pollut., 2009, 157(2), 371—376 |
| [13] | Holmes L., Turner A., Environ. Pollut., 2009, 157(12), 3440—3444 |
| [14] | Singh N., Turner A., Mar. Pollut. Bull., 2009, 58(4), 559—564 |
| [15] | Voulvoulis N., Scrimshaw M. D., Lester J. N., Appl. Organomet. Chem., 1999, 13(3), 135—143 |
| [16] | Almeida E., Diamantino T. C., de Sousa O., Prog. Org. Coat., 2007, 59(1), 2—20 |
| [17] | Hao X. P., Chen S. G., Yu H., Liu D., Sun W. X., RSC Adv., 2016, 6(1), 39—43 |
| [18] | Shtylova L., Fant C., Handa P., Larsson A., Berntsson K., Blanck H., Simonsson R., Nyden M., Haerelind H. I., Prog. Org. Coat., 2009, 64(1), 20—26 |
| [19] | Fant C., Handa P., Nyden M., J. Phys. Chem. B, 2006, 110(43), 21808—21815 |
| [20] | Sancet M. P. A., Hanke M., Wang Z., Bauer S., Azucena C., Arslan H. K., Heinle M., Gliemann H., Woell C., Rosenhahn A., Biointerphases, 2013, 8(29), 1—8 |
| [21] | Nishat N., Dhyani S., Hasnain S., Manisha Polym. Bull., 2010, 64(6), 523—536 |
| [22] | Ahamad T., Kumar V., Parveen S., Nishat N., Appl. Organomet. Chem., 2007, 21(12), 1013—1021 |
| [23] | Mahmoud W. H., Deghadi R. G., Mohamed G. G., Appl. Organomet. Chem., 2016, 30(4), 221—230 |
| [24] | Rasool R., Hasnain S., Iran. Polym. J., 2015, 24(10), 891—900 |
| [25] | Gitlitz M. H., Leiner H. H., Erodible Shop-bottom Paints for Control of Marine Fouling, US4593055,1986-06-03 |
| [26] | Wu K. H., Chang T. C., Wang Y. T., Hong Y. S., Wu T. S., Eur. Polym. J. , 2003, 39(2), 239—245 |
| [27] | Pekel N., Guner A., Guven O., J. Appl. Polym. Sci., 2002, 85(2), 376—384 |
| [28] | Xiong Z.J., Li X. N., Jia Q. X., Fu Z. Y, Yang Z. K.,Acta Polym. Sin., 2010, (8), 1003—1008 |
| (熊祖江, 李小宁, 贾清秀, 付中玉, 杨中开. 高分子学报, 2010, (8), 1003—1008) |
| [1] | GE Yicong, NIE Wanli, SUN Guofeng, CHEN Jiaxuan, TIAN Chong. Silver-catalyzed [5+1] Cyclization of 2-Vinylanilines with Benzisoxazoles [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220142. |
| [2] | YAN Shuting, YAO Yuan, TAO Xinfeng, LIN Shaoliang. Synthesis and Properties of Polypeptoid Hydrogels Containing Sulfonium Groups [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220381. |
| [3] | YAN Tingting, ZHANG Na, LI Qiang, LI Zhenhua, LI Chunhui, LI Xue, YU Ru, WANG Rui, WANG Jihua, CAO Zanxia. Effects of Co-reagent for Improving the Performance of Polyamide Composite Reverse Osmosis Membrane [J]. Chem. J. Chinese Universities, 2021, 42(6): 2008. |
| [4] | WANG Ruxin, ZHAO Zhongjun, HE Feiyao, YUE Hanlu, DENG Fulong, LI Hong, LI Wenwen, DUAN Yixiang. Characteristic Analysis of C1—C3n-Aldehydes and n-Alcohols in Proton Transfer Reaction Time-of-flight Mass Spectrometry [J]. Chem. J. Chinese Universities, 2021, 42(12): 3632. |
| [5] | WANG Yiqiao,WANG Cong,LIU Xingman,ZHANG Min,GENG Yun,ZHAO Liang. Planar Tetracoordinate Boron and Pentacoordinate Boron in B6S5 Clusters† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1625. |
| [6] | YAN Hao, TANG Ping, LI Shuhong, ZHAO Tianyi, LIU Mingjie. Progress of Biomimetic Anisotropic Poly(N-isopropylacrylamide) Intelligent Response Actuators † [J]. Chem. J. Chinese Universities, 2020, 41(5): 936. |
| [7] | SO Chauming, YUEN Onying, KWONG Fukyee, CHEN Chihchiang, PAI Chengchao, SUN Raymond Waiyin. Application of CM-Phos Ligand in Palladium-catalyzed Cross-coupling Reactions† [J]. Chem. J. Chinese Universities, 2020, 41(10): 2185. |
| [8] | ZHANG Nanxi, LV Jingwei, JIN Ping, LI Jingfeng, BIAN Xuefeng, ZHANG Hui, SUN Jiaming. 1H NMR Metabonomic Investigations of N-Benzylhexadecanamide Induced Proliferation and Iestosterone Secretion of Mouse Testicular Leydig Cells† [J]. Chem. J. Chinese Universities, 2019, 40(9): 1832. |
| [9] | DONG Lirong,WANG Siyu,ZHANG Xiaomei,CHENG Jiajia,YUAN Yaofeng. High Efficiency Catalytic Synthesis of N-Sulfonyltriazole in Aqueous Phase by Copper Sulfate/Substituted Thiourea† [J]. Chem. J. Chinese Universities, 2019, 40(5): 927. |
| [10] | CAI Yanchao,NIU Pengfei,SHEN Zhenlu,LI Meichao. Electrocatalytic Oxidative Coupling of Primary Amines with the Medium ABNO † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2308. |
| [11] | FANG Sheng, WANG Meiyan, LIU Jingjing, LIU Jingyao. Reaction Mechanism of Nickel Complex Catalyzed Isomerization of N-Allylamides† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1475. |
| [12] | TONG Bo, ZHANG Zhongxiang, LIU Zhenjie, PENG Zhangquan, ZHOU Zhibin. Novel Electrolyte Containing Li[(CF3SO2)(n-C4F9SO2)N] for High Voltage LiNi0.5Mn1.5O4-based Cell† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1518. |
| [13] | YI Junming,SONG Sen,ZHANG Sheng,ZHANG Shaowei,TIAN Mengkui,NI Xinlong. Effects of Terminal Groups of Guest on the Pseudorotaxane Assembly of Cucurbit[7]uril† [J]. Chem. J. Chinese Universities, 2018, 39(5): 911. |
| [14] | YANG Qinghua, WANG Longgang, LIU Jie, LU Yong, CHEN Tianyun. Preparation and Characterization of Star-shaped β-Cyclodextrin Based Polymer† [J]. Chem. J. Chinese Universities, 2018, 39(4): 793. |
| [15] | LI Lingmei, WANG Chengjian, HAN Jianli, LIU Rendan, WEN Yanan, WEI Ming, JIN Wanjun, HUANG Linjuan, WANG Zhongfu. Mass Spectrometric Analysis of N-Glycosylation Sites and N-Glycans of β-Conglycinin† [J]. Chem. J. Chinese Universities, 2018, 39(3): 427. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||