

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (1): 12.doi: 10.7503/cjcu20160647
• Articles: Inorganic Chemistry • Previous Articles Next Articles
XU Zhongxuan*( ), ZHAO Guanglian, LUO Le, LONG Zhenmin
), ZHAO Guanglian, LUO Le, LONG Zhenmin
Received:2016-09-19
Online:2017-01-10
Published:2016-12-20
Contact:
XU Zhongxuan
E-mail:xuzhongxuan4201@163.com
Supported by:CLC Number:
TrendMD:
XU Zhongxuan, ZHAO Guanglian, LUO Le, LONG Zhenmin. Synthesis, Structures and Properties of Homochiral Coordination Polymers Assembled from Semirigid Lactic Acid Derivatives†[J]. Chem. J. Chinese Universities, 2017, 38(1): 12.
| Compound | 1-D | 1-L | 2-D | 2-L |
|---|---|---|---|---|
| Chemical formula | C32H30Cd2N2O12 | C32H30Cd2N2O12 | C22H20CdN4O6 | C22H20CdN4O6 |
| Molecular weight | 859.41 | 859.41 | 548.83 | 548.83 |
| Crystal system | Triclinic | Triclinic | Orthorhmbic | Orthorhmbic |
| a/nm | 0.79969(4) | 0.79362(3) | 1.61472(5) | 1.61199(9) |
| b/nm | 0.94480(6) | 0.93695(4) | 1.68334(6) | 1.68962(11) |
| c/nm | 1.11730(7) | 1.11630(4) | 8.3019(3) | 8.3055(5) |
| α/(°) | 87.681(5) | 87.855(3) | 90 | 90 |
| β/(°) | 75.203(4) | 75.212(3) | 90 | 90 |
| γ/(°) | 71.788(5) | 72.171(4) | 90 | 90 |
| Unit cell volume/nm3 | 0.77458(8) | 0.76327(6) | 2.25656(13) | 2.2621(2) |
| Temperature/K | 295.27(10) | 293(2) | 293(2) | 293(2) |
| Space group | P1 | P1 | P21212 | P21212 |
| No. of formula units per unit cell, Z | 1 | 1 | 4 | 4 |
| Radiation type | Cu Kα | Mo Kα | Mo Kα | Mo Kα |
| Absorption coefficient, μ/mm-1 | 11.605 | 1.464 | 1.014 | 1.011 |
| No. of reflections measured | 4800 | 11740 | 6672 | 16928 |
| No. of independent reflections | 3283 | 6688 | 4086 | 4840 |
| Rint | 0.0385 | 0.0258 | 0.0310 | 0.0249 |
| Final R1/wR(F2)values[I > 2σ(I)] | 0.0562/0.1755 | 0.0264/0.0515 | 0.0411/0.0643 | 0.0258/0.0626 |
| Final R1/wR(F2) values(all data) | 0.0569/0.1766 | 0.0297/0.0529 | 0.0546/0.0681 | 0.0287/0.0648 |
| Goodness of fit on F2 | 1.059 | 0.894 | 1.006 | 1.002 |
| Flack parameter | 0.020(14) | -0.063(17) | -0.04(2) | 0.018(9) |
| CCDC No. | 1502336 | 1502337 | 1502338 | 1502339 |
Table 1 Crystal data and refinement results for complexes 1-D, 1-L, 2-D and 2-L
| Compound | 1-D | 1-L | 2-D | 2-L |
|---|---|---|---|---|
| Chemical formula | C32H30Cd2N2O12 | C32H30Cd2N2O12 | C22H20CdN4O6 | C22H20CdN4O6 |
| Molecular weight | 859.41 | 859.41 | 548.83 | 548.83 |
| Crystal system | Triclinic | Triclinic | Orthorhmbic | Orthorhmbic |
| a/nm | 0.79969(4) | 0.79362(3) | 1.61472(5) | 1.61199(9) |
| b/nm | 0.94480(6) | 0.93695(4) | 1.68334(6) | 1.68962(11) |
| c/nm | 1.11730(7) | 1.11630(4) | 8.3019(3) | 8.3055(5) |
| α/(°) | 87.681(5) | 87.855(3) | 90 | 90 |
| β/(°) | 75.203(4) | 75.212(3) | 90 | 90 |
| γ/(°) | 71.788(5) | 72.171(4) | 90 | 90 |
| Unit cell volume/nm3 | 0.77458(8) | 0.76327(6) | 2.25656(13) | 2.2621(2) |
| Temperature/K | 295.27(10) | 293(2) | 293(2) | 293(2) |
| Space group | P1 | P1 | P21212 | P21212 |
| No. of formula units per unit cell, Z | 1 | 1 | 4 | 4 |
| Radiation type | Cu Kα | Mo Kα | Mo Kα | Mo Kα |
| Absorption coefficient, μ/mm-1 | 11.605 | 1.464 | 1.014 | 1.011 |
| No. of reflections measured | 4800 | 11740 | 6672 | 16928 |
| No. of independent reflections | 3283 | 6688 | 4086 | 4840 |
| Rint | 0.0385 | 0.0258 | 0.0310 | 0.0249 |
| Final R1/wR(F2)values[I > 2σ(I)] | 0.0562/0.1755 | 0.0264/0.0515 | 0.0411/0.0643 | 0.0258/0.0626 |
| Final R1/wR(F2) values(all data) | 0.0569/0.1766 | 0.0297/0.0529 | 0.0546/0.0681 | 0.0287/0.0648 |
| Goodness of fit on F2 | 1.059 | 0.894 | 1.006 | 1.002 |
| Flack parameter | 0.020(14) | -0.063(17) | -0.04(2) | 0.018(9) |
| CCDC No. | 1502336 | 1502337 | 1502338 | 1502339 |
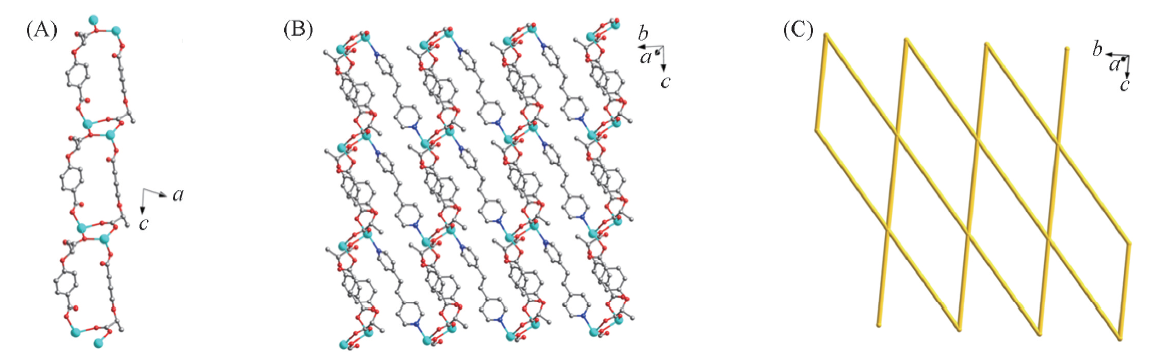
Fig.2 1D ladder-like chain constructed by Cd2+ ions and (R)-CBA2- ligands(A), 2D framework of 1-D composed of 1D Cd-(R)-CBA2- chains and DPEE ligands(B) and the sql net of 1-D(C)
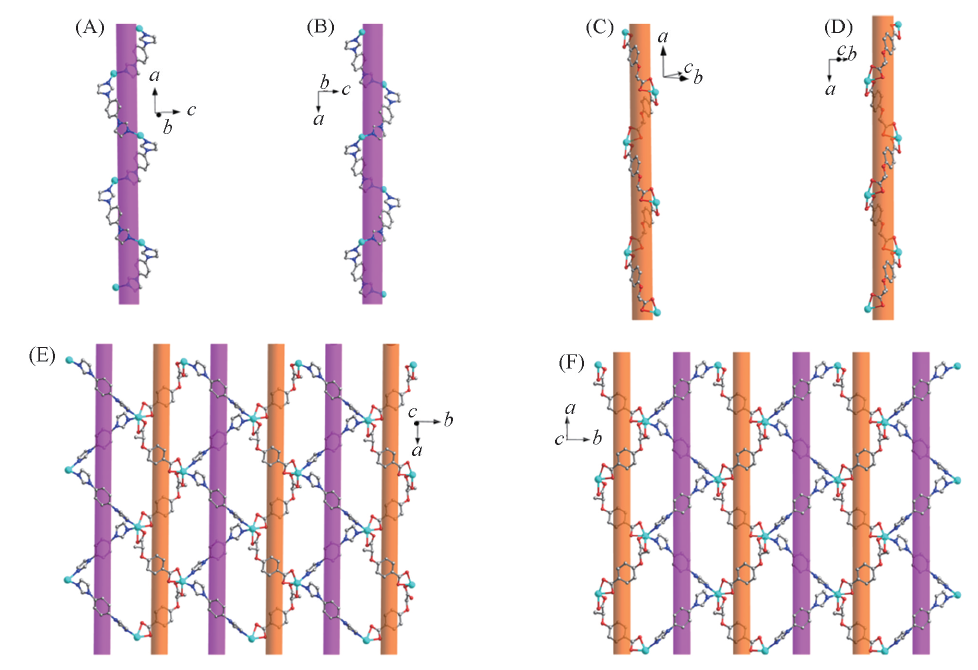
Fig.4 Left-handed helical A-chain in 2-D(A), right-handed helical A-chain in 2-L(B), left-handed helical B-chain in 2-D(C), right-handed helical B-chain in 2-L(D), 2D layer of 2-D constructed by left-handed helical A-chains and B-chains(E) and 2D layer of 2-L constructed by right-handed helical A-chains and B-chains(F)
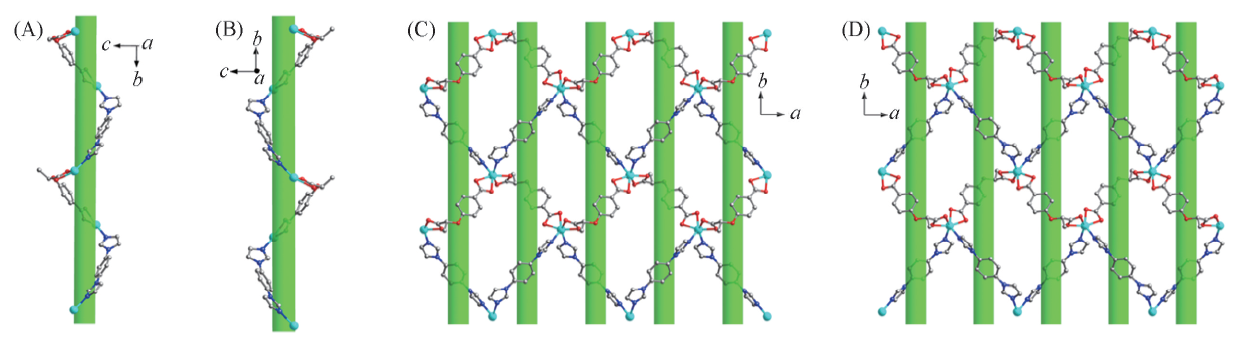
Fig.5 Right-handed helical C-chain in 2-D(A), left-handed helical C-chain in 2-L(B), 2D layer of 2-D constructed by right-handed helical C-chains(C) and 2D layer of 2-L constructed by right-handed helical C-chains(D)
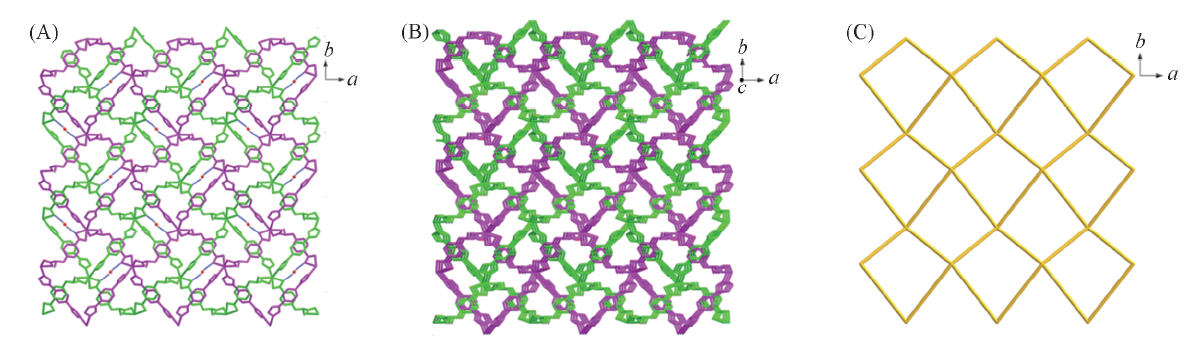
Fig.6 AB type double-layered framework of 2-D linked by hydrogen bonds(A), 3D supramolecular framework of 2-D(B) and the 4-connected sql net in 2-D(C)
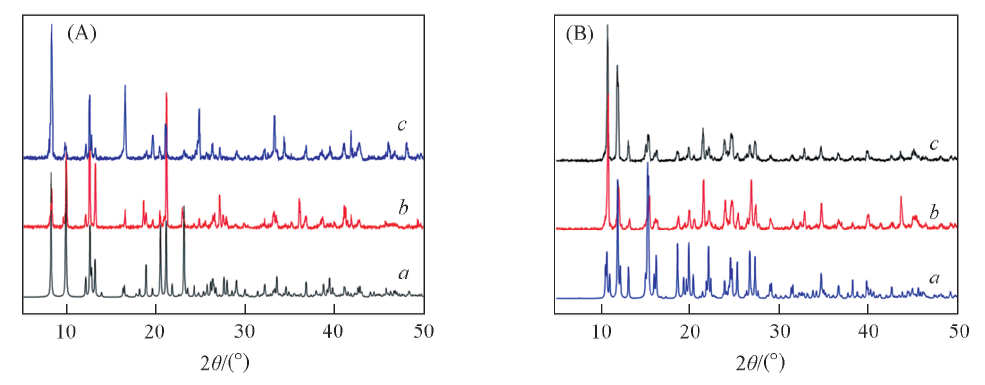
Fig.7 PXRD patterns of complexes 1(A) and 2(B)(A) a. Simulated, 1-D, b. experimental, 1-D, c. experimental, 1-L; (B) a. simulated, 2-D, b. experimental, 2-D, c. experimental, 2-L.
| [1] | Duarte M., Billing J., Yilmaz E.,J. Appl. Polym. Sci., 2016, DOI: 10.1002/APP.44104 |
| [2] | Shen J., Okamoto Y., Chem. Rev., 2016, 116, 1094—1138 |
| [3] | Fuchs I., Fechler N., Antonietti M., Mastai Y., Angew. Chem. Int. Ed., 2016, 55, 408—412 |
| [4] | Li W., Wang B., Yang W., Deng J., Macromol. Rapid Commun., 2015, 36, 319—326 |
| [5] | Zhang L., Qin L., Wang X. F., Cao H., Liu M. H., Adv. Mater., 2014, 26, 6959—6964 |
| [6] | Kelly J. A., Giese M., Shopsowitz K. E., Maclachlan M. J., Acc. Chem. Res., 2014, 47, 1088—1096 |
| [7] | Yoon M., Srirambalaji R., Kim K., Chem. Rev., 2012, 112, 1196—1231 |
| [8] | Ma L., Abney C., Lin W. B., Chem. Soc. Rev., 2009, 38, 1248—1256 |
| [9] | Xi X. B., Fang Y., Dong T. W., Cui Y., Angew. Chem., Int. Ed., 2011, 50, 1154—1158 |
| [10] | Mo K., Yang Y. H., Cui Y., J. Am. Chem. Soc., 2014, 136, 1746—1749 |
| [11] | Peng Y. W., Gong T. F., Cui Y., Chem. Commun., 2013, 49, 8253—8255 |
| [12] | Liu Y., Xi X. B., Ye C. C., Gong T. F., Yang Z. W., Cui Y., Angew. Chem. Int. Ed., 2014, 53, 13821—13825 |
| [13] | Wu P. Y., He C., Wang J., Peng X. J., Li X. Z., An Y. L., Duan C. Y., J. Am. Chem. Soc., 2012, 134, 14991—14999 |
| [14] | Hang Q. X., He C., Zhao M., Qi B., Niu J. Y., Duan C. Y., J. Am. Chem. Soc., 2013, 135, 10186—10189 |
| [15] | Han Q.X., Qi B., Ren W. M., He C., Niu J. Y., Duan C. Y.,Nat. Commun., 2016, DOI: 10.1038/ncomms10007 |
| [16] | Kuang X., Ma Y., Su H., Zhang J., Dong Y. B., Tang B., Anal. Chem., 2014, 86, 1277—1281 |
| [17] | Zhou C., Wen Y. H., Wu X. T., CrystEngComm., 2016, 18, 2792—2802 |
| [18] | Wen H. R., Xie R. X., Liu S. J., Bao J., Wang F. F., Liu C. M., Liao J. S., RSC Adv., 2015, 5, 98097—98104 |
| [19] | Bhunia A., Dey S., Moreno J. M., Diaz U., Concepcion P., Hecke K. V., Janiak C., Voort P. V. D., Chem. Commun., 2016, 52, 1401—1404 |
| [20] | Ma L. Q., Lin W. B., J. Am. Chem. Soc., 2008, 130, 13834—13835 |
| [21] | Chang C. L., Qi X. Y., Zhang J. W., Qiu Y. M., Li X. J., Wang X., Bai Y., Sun J. L., Liu H. W., Chem. Commun., 2015, 51, 3566—3569 |
| [22] | Yuan S., Deng Y. K., Xuan W. M., Wang X. P., Wang S. N., Dou J. M., Sun D., Cryst. Eng. Comm,2014, 16, 3829—3833 |
| [23] | Feng J. S., Ren M., Cai Z. S., Fan K., Bao S. S., Zheng L. M., Chem. Commun., 2016, 52, 6877—6880 |
| [24] | Mihalcea I., Zill N., Mereacre V., Anson C. E., Powell A. K., Cryst. Growth Des., 2014, 14, 4729—4734 |
| [25] | Sun J. W., Zhu J., Song H. F., Li G. M., Yao X., Yan P. F., Cryst. Growth Des., 2014, 14, 5356—5360 |
| [26] | Jeong K. S., Go Y. B., Shin S. M., Lee S. J., Kim J., Yaghi O. M., Jeong N., Chem. Sci., 2011, 2, 877—882 |
| [27] | Lun D. J., Waterhouse G. I. N., Telfer S. G., J. Am. Chem. Soc., 2011, 133, 5806—5809 |
| [28] | Regati S., He Y., Thimmaiah M., Li P., Xiang S., Chen B., Zhao C. G., Chem. Commun., 2013, 49, 9836—9838 |
| [29] | Zhao X., Wong M., Mao C., Trieu T. X., Zhang J., Feng P. Y., Bu X., J. Am. Chem. Soc., 2014, 136, 12572—12575 |
| [30] | Kuang X., Ye S., Li X., Ma Y., Zhang C., Tang B., Chem. Commun., 2016, 52, 5432—5435 |
| [31] | Yang X. L., Wu C. D., Cryst. Eng. Comm., 2014, 16, 4907—4918 |
| [32] | Kumar N., Khullar S., Singh Y., Mandal S. K., Cryst. Eng. Comm., 2014, 16, 6730—6744 |
| [33] | Chen L., Kang J., Cui H., Wang Y., Liu L., Zhang L., Su C. Y., Dalton Trans., 2015, 44, 12180—12188 |
| [34] | Bisht K. K., Parmar B., Rachuri Y., Kathalikattil A. C., Suresh E., Cryst. Eng. Comm., 2015, 17, 5341—5356 |
| [35] | Xu Z. X., Tan Y. X., Fu H. R., Liu J., Zhang J., Inorg. Chem., 2014, 53, 12199—12204 |
| [36] | Xu X. Z., Tan Y. X., Fu H. R., Kan Y., Zhang J., Chem. Commun., 2015, 51, 2565—2568 |
| [37] | Xu Z. Xu., Xiao Y., Kang Y., Zhang L., Zhang J., Cryst. Growth Des., 2015, 15, 4676—4686 |
| [38] | Cui P., Chen Z., Gao D. L., Zhao B., Shi W., Cheng P., Cryst. Growth Des., 2010, 10, 4370—4378 |
| [39] | Cui Y. J., Yue Y. F., Qian G. D., Chen B. L., Chem. Rev., 2012, 112, 1126—1162 |
| [40] | Hu Z. C., Deiber B. J., Li J., Chem. Soc. Rev., 2014, 43, 5815—5840 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||