

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (2): 215.doi: 10.7503/cjcu20140957
• Articles: Inorganic Chemistry • Previous Articles Next Articles
MA Benhua, PENG Yu, HUA Jia, SHI Zhan*( )
)
Received:2014-10-29
Online:2015-02-10
Published:2015-01-22
Contact:
SHI Zhan
E-mail:zshi@mail.jlu.edu.cn
Supported by:CLC Number:
TrendMD:
MA Benhua, PENG Yu, HUA Jia, SHI Zhan. Synthesis and Characterization of Coordination Polymers Based on 5-Azidoisophthalic Acid†[J]. Chem. J. Chinese Universities, 2015, 36(2): 215.
| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C23H18CoN6O4 | C18H11CoN5O4 | C18H11MnN5O4 | C20H11MnN9O4 |
| Formula weight | 501.36 | 420.25 | 416.26 | 496.32 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Triclinic |
| Space group | C2/c | C2/m | C2/m | P |
| a/nm | 2.0952(12) | 1.8070(4) | 1.8296(4) | 0.9982(2) |
| b/nm | 1.5897(12) | 1.1587(2) | 1.1613(2) | 1.0215(2) |
| c/nm | 1.4735(9) | 1.0182(2) | 1.0196(2) | 1.4518(3) |
| α/(°) | 90 | 90 | 90 | 81.58(3) |
| β/(°) | 112.21(5) | 109.42(3) | 109.57(3) | 76.89(3) |
| γ/(°) | 90 | 90 | 90 | 71.99(3) |
| Volume/nm3 | 4.5437(5) | 2.0106(7) | 2.0411(7) | 1.3665(5) |
| Z | 8 | 4 | 4 | 2 |
| Calculated density/(g·cm-3) | 1.466 | 1.388 | 1.355 | 1.206 |
| Absorption coefficient/mm-1 | 0.798 | 0.886 | 0.678 | 0.522 |
| F(000) | 2056 | 852 | 844 | 502 |
| GOF | 1.021 | 1.113 | 1.093 | 1.067 |
| Final R index[I>2σ(I)] | R1=0.0372 | R1=0.0817 | R1=0.0766 | R1=0.0819 |
| wR2=0.0882 | wR2=0.2422 | wR2=0.2398 | wR2=0.2676 | |
| R index(All data) | R1=0.0844 | R1=0.0996 | R1=0.0995 | R1=0.1252 |
| wR2=0.1098 | wR2=0.2564 | wR2=0.2535 | wR2=0.2938 |
Table 1 Crystallographic data for complexes 1—4
| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C23H18CoN6O4 | C18H11CoN5O4 | C18H11MnN5O4 | C20H11MnN9O4 |
| Formula weight | 501.36 | 420.25 | 416.26 | 496.32 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Triclinic |
| Space group | C2/c | C2/m | C2/m | P |
| a/nm | 2.0952(12) | 1.8070(4) | 1.8296(4) | 0.9982(2) |
| b/nm | 1.5897(12) | 1.1587(2) | 1.1613(2) | 1.0215(2) |
| c/nm | 1.4735(9) | 1.0182(2) | 1.0196(2) | 1.4518(3) |
| α/(°) | 90 | 90 | 90 | 81.58(3) |
| β/(°) | 112.21(5) | 109.42(3) | 109.57(3) | 76.89(3) |
| γ/(°) | 90 | 90 | 90 | 71.99(3) |
| Volume/nm3 | 4.5437(5) | 2.0106(7) | 2.0411(7) | 1.3665(5) |
| Z | 8 | 4 | 4 | 2 |
| Calculated density/(g·cm-3) | 1.466 | 1.388 | 1.355 | 1.206 |
| Absorption coefficient/mm-1 | 0.798 | 0.886 | 0.678 | 0.522 |
| F(000) | 2056 | 852 | 844 | 502 |
| GOF | 1.021 | 1.113 | 1.093 | 1.067 |
| Final R index[I>2σ(I)] | R1=0.0372 | R1=0.0817 | R1=0.0766 | R1=0.0819 |
| wR2=0.0882 | wR2=0.2422 | wR2=0.2398 | wR2=0.2676 | |
| R index(All data) | R1=0.0844 | R1=0.0996 | R1=0.0995 | R1=0.1252 |
| wR2=0.1098 | wR2=0.2564 | wR2=0.2535 | wR2=0.2938 |
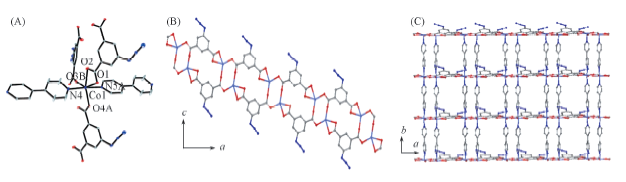
Fig.2 Crystal structure of complex 2(A), view of the 1D chain structure of [Co2(aip)2]n along a and c axes(B) and the view of the 2D layer structure along a and b axes(C)
| [1] | Eddaoudi M., Moler D. B., Reineke T. M., O'Keeffe M., Yaghi O. M., Acc. Chem. Res., 2001, 34, 319—330 |
| [2] | Ferey G., Draznieks C. M., Millange F., Acc. Chem. Res., 2005, 38, 217—225 |
| [3] | Luo T. T., Wu H. C., Jao Y. C., Huang S. M., Tseng T. W., Wen Y. S., Lee G. H., Peng S. M., Lu K. L., Angew. Chem. Int. Ed., 2009, 48, 9461—9464 |
| [4] | Eddaoudi M., Kim J., Rosi N., Vodak D., Wachter J., O'Keeffe M., Yaghi O., Science, 2002, 295, 469—472 |
| [5] | Perry IV J. J., Perman J. A., Zaworotko M. J., Chem. Soc. Rev., 2009, 38, 1400—1417 |
| [6] | Lu W. G., Liu H. W., Yin X. G., Lan L. M., Liu H. Y., Chem. J. Chinese Universities, 2013, 34(12), 2691—2698 |
| (卢文贯, 刘宏文, 殷旭光, 蓝路梅, 刘海瑶. 高等学校化学学报, 2013, 34(12), 2691—2698) | |
| [7] | Evans O. R., Lin W. B., Acc. Chem. Res., 2002, 35, 511—522 |
| [8] | Stylianou K. C., Heck R., Chong S. Y., Bacsa J., Jones J. T. A., Khimyak Y. Z., Bradshaw D., Rossenisky M. J., J. Am. Chem. Soc., 2010, 132, 4119—4130 |
| [9] | Shi W. B., Kou H. Z., Chem. J. Chinese Universities, 2014, 35(1), 12—18 |
| (石文博, 寇会忠. 高等学校化学学报, 2014, 35(1), 12—18) | |
| [10] | Luisi B.S., Rowland K. D., Moulton B.,Chem. Common., 2007, 2802—2804 |
| [11] | Alkordi M. H., Liu Y., Larsen R. W., Eubank J. F., Eddaoudi M., J. Am. Chem. Soc., 2008, 130, 12639—12641 |
| [12] | Cai K., Zhang L. N., Han L. Q., Qu F. Y., Chem. J. Chinese Universities, 2013, 34(6), 1313—1317 |
| (蔡锟, 张玲娜, 韩丽琴, 曲凤玉. 高等学校化学学报, 2013, 34(6), 1313—1317) | |
| [13] | Sumida K., Horike S., Kaye S. S., Herm Z. R., Queen W. L., Brown C. M., Grandjean F., Long G. J., Dailly A., Long J. R., Chem. Sci., 2010, 1, 184—191 |
| [14] | Xu G., Zhang X., Guo P., Pan C., Zhang H., Wang C., J. Am. Chem. Soc., 2010, 132, 3656—3657 |
| [15] | Cui Y., Yue Y., Qian G., Chen B., Chem. Rev., 2012, 112, 1126—1162 |
| [16] | Li Z. Q., Wang A., Guo C. Y., Hu W. N., Tai Y. F., Chem. J. Chinese Universities, 2013, 34(11), 2470—2477 |
| (李宗群, 汪艾, 郭春燕, 胡文娜, 邰燕芳. 高等学校化学学报, 2013, 34(11), 2470—2477) | |
| [17] | Furukawa H., Ko N., Go Y. B., Aratani N., Choi S. B., Choi E., Yazaydin A., Snurr R. Q., O'keeffe M., Kim J., Yaghi O. M., Science, 2010, 329, 424—428 |
| [18] | Rocca J. D., Liu D., Lin W., Acc. Chem. Res., 2011, 44, 957—968 |
| [19] | Tranchemontagne D. J., Cortes J. L. M., O'keeffe M., Yaghi O. M., Chem. Soc. Rev., 2009, 38, 1257—1283 |
| [20] | Wang W., Xu L., Gao G., Li F., Liu X., Liu L., Inorg. Chem. Commun., 2009, 12, 875—878 |
| [21] | Gao Q., Jiang F. L., Wu M. Y., Huang Y. G., Yuan D. Q., Wei W., Hong M. C., Cryst. Eng. Comm., 2009, 11, 918—926 |
| [22] | Bhattacharya S., Goswami A., Gole B., Ganguly S., Bala S., Sengupta S., Khanra S., Mondal R., Cryst. Grow. Des., 2014, 14, 2853—2865 |
| [23] | Cui J., Yang Q., Li Y., Guo Z., Zheng H., Cryst. Grow. Des., 2013, 13, 1694—1702 |
| [24] | Sato H., Matsuda R., Sugimoto K., Takata M., Kitagawa S., Nature Mater., 2010, 9, 661—666 |
| [25] | Farha O. K., Malliakas C. D., Kanatzidis M. G., Hupp J. T., J. Am. Chem. Soc., 2010, 132, 950—952 |
| [26] | Bruker AXS Inc., SHELXTL, Madison, 2000 |
| (Ed.: F, K, M) |
| [1] | LIU Qingqing, WANG Pu, WANG Yongshuai, ZHAO Man, DONG Huanli. Synthesis and Topochemical Polymerization Study of Naphthalene/perylene Imides Substituted Diacetylene Derivatives [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220091. |
| [2] | SHI Naike, ZHANG Ya, SANSON Andrea, WANG Lei, CHEN Jun. Uniaxial Negative Thermal Expansion and Mechanism in Zn(NCN) [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220124. |
| [3] | LU Cong, LI Zhenhua, LIU Jinlu, HUA Jia, LI Guanghua, SHI Zhan, FENG Shouhua. Synthesis, Structure and Fluorescence Detection Properties of a New Lanthanide Metal-Organic Framework Material [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220037. |
| [4] | MA Jianxin, LIU Xiaodong, XU Na, LIU Guocheng, WANG Xiuli. A Multi-functional Zn(II) Coordination Polymer with Luminescence Sensing, Amperometric Sensing, and Dye Adsorption Performance [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210585. |
| [5] | YUE Shengli, WU Guangbao, LI Xing, LI Kang, HUANG Gaosheng, TANG Yi, ZHOU Huiqiong. Research Progress of Quasi-two-dimensional Perovskite Solar Cells [J]. Chem. J. Chinese Universities, 2021, 42(6): 1648. |
| [6] | YU Qiangmin, ZHANG Zhiyuan, LUO Yuting, LI Yang, CHENG Huiming, LIU Bilu. Solvothermal Synthesis of 2D Metallic Transition Metal Disulfides for Efficient Electrocatalytic Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2021, 42(2): 654. |
| [7] | ZHANG Junying, PENG Wei, CHEN Ziwei, HE Aihua. Effect of the Polymerization Temperature on the Copolymerization of Butadiene and Isoprene Catalyzed by Supported Ziegler-Natta Catalyst [J]. Chem. J. Chinese Universities, 2020, 41(8): 1873. |
| [8] | TIAN Xia,YANG Fuqun,YUAN Wei,ZHAO Lei,YAO Lei,ZHEN Xiaoli,HAN Jianrong,LIU Shouxin. Synthesis, Structure and Recognition Properties of Macrocyclic Crown Ethers with Oxadiazole † [J]. Chem. J. Chinese Universities, 2020, 41(3): 490. |
| [9] | LIU Dongmei,SU Yajing,LI Shanshan,XU Qiwei,LI Xia. Transition Metal Coordination Polymers Constructed by 4-(4-Carboxyphenoxy)isophthalic Acid: Synthesis, Crystal Structure, Fluorescence Sensing and Photocatalysis † [J]. Chem. J. Chinese Universities, 2020, 41(2): 253. |
| [10] | XIAN Guoxuan, YU Yu’e, CHEN Yuqian, WAN Xiaoyu, WANG Suna, LU Jing. Coligand Induced Luminescent Cd-MOFs: Luminescence Enhancement Toward Acetylacetone and Quenching Toward Cr2O72- [J]. Chem. J. Chinese Universities, 2020, 41(12): 2725. |
| [11] | QIN Liulei,LIU Yang,GUAN Xiaoqin,ZHENG Xiaoyuan,ZHANG Ziyu,LIU Zunqi. Synthesis and Switchable Dielectric Properties of an Inorganic-organic Hybrid Complex [H2(DABCO)CuCl4]·H2O † [J]. Chem. J. Chinese Universities, 2020, 41(1): 70. |
| [12] | LI Bing,WANG Xuemin,BAI Fengying,LIU Shuqing. Synthesises, Structures and Antibacterial Activities of a Series of Rare Earth Nitrogen Heterocyclic Complexes† [J]. Chem. J. Chinese Universities, 2019, 40(4): 632. |
| [13] | Baozhen SHI,Shan LI,Dianpeng WANG,Yunzhi ZHOU,Jinyu SUN. Synthesis and Physical Properties of Cobalt-Zinc Hybrid Porous Metal-organic Frameworks † [J]. Chem. J. Chinese Universities, 2019, 40(12): 2443. |
| [14] |
JIANG Xianming,WANG Huaiqian,CAO Yu,SUN Zhihui,CAO Yufang,WU Weibin.
Structure Prediction and Photoelectron Spectroscopy Study of Rare Earth-doped Silicon-based Clusters of MS |
| [15] | WANG Dongmei,LIU Zihua,LI Guanghua,LIU Yunling,LI Chunxia. Synthesis, Structure and Fluorescent Property of Indium-based Bimetallic Metal-organic Frameworks† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1886. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||