

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (7): 1584.doi: 10.7503/cjcu20140076
• Polymer Chemistry • Previous Articles Next Articles
DUAN Cuijia1,2, CAO Yiming1,*( ), JIE Xingming1, WANG Lina1, YUAN Quan1
), JIE Xingming1, WANG Lina1, YUAN Quan1
Received:2014-01-23
Online:2014-07-10
Published:2014-05-12
Contact:
CAO Yiming
E-mail:ymcao@dicp.ac.cn
CLC Number:
TrendMD:
DUAN Cuijia, CAO Yiming, JIE Xingming, WANG Lina, YUAN Quan. Preparation and Gas Separation Properties of Metal-organic Frameworks/Polyimide Mixed Matrix Membranes†[J]. Chem. J. Chinese Universities, 2014, 35(7): 1584.
| MOFs | BET surface/(m2·g-1) | Pore volume/(cm3·g-1) | Particle size/μm |
|---|---|---|---|
| Cu3(BTC)2 | 1439 | 0.642 | 2—9 |
| S-Cu3(BTC)2 | 171.4 | 0.154 | 0.1—1.0 |
| ZIF-8 | 1653 | 0.592 | 0.3—1.0 |
Table 1 Structure properties of Cu3(BTC)2, S-Cu3(BTC)2 and ZIF-8
| MOFs | BET surface/(m2·g-1) | Pore volume/(cm3·g-1) | Particle size/μm |
|---|---|---|---|
| Cu3(BTC)2 | 1439 | 0.642 | 2—9 |
| S-Cu3(BTC)2 | 171.4 | 0.154 | 0.1—1.0 |
| ZIF-8 | 1653 | 0.592 | 0.3—1.0 |
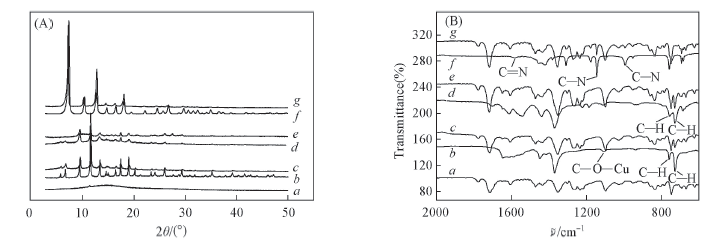
Fig.3 XRD patterns(A) and FTIR-ATR spectra(B) of ODPA-TMPDA membrane, MOFs and MOFs/ODPA-TMPDA mixed matrix membranes a. ODPA-TMPDA membrane; b. Cu3(BTC)2; c. Cu3(BTC)2/ODPA-TMPDA MMM; d. S-Cu3(BTC)2;
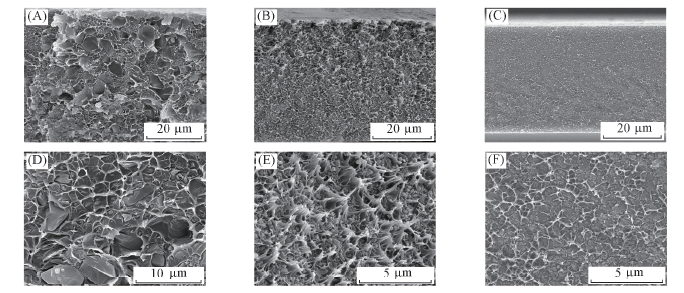
Fig.4 Cross-section SEM images of MOFs/ODPA-TMPDA mixed matrix membranes at low(A—C) and high(D—F) magnification (A),(D) Cu3(BTC)2/ODPA-TMPDA MMM; (B),(E) S-Cu3(BTC)2/ODPA-TMPDA MMM; (C),(F) ZIF-8/ODPA-TMPDA MMM.
| Membrane | P/Barrer | Selectivity | |||||
|---|---|---|---|---|---|---|---|
| O2 | N2 | CH4 | CO2 | O2/N2 | CO2/CH4 | ||
| ODPA-TMPDA | 8.93 | 1.74 | 1.63 | 47.76 | 5.13 | 29.30 | |
| Cu3(BTC)2/ODPA-TMPDA | 35.47 | 7.07 | 7.00 | 180.80 | 5.02 | 26.64 | |
| S-Cu3(BTC)2/ODPA-TMPDA | 25.00 | 5.27 | 4.94 | 131.70 | 4.93 | 26.30 | |
| ZIF-8/ODPA-TMPDA | 38.79 | 8.90 | 7.71 | 189.80 | 4.81 | 23.53 | |
| Maxwell model | 20.41 | 3.98 | 3.73 | 109.20 | 5.13 | 29.30 | |
| Bruggeman model | 26.03 | 5.07 | 4.75 | 139.20 | 5.13 | 29.30 | |
Table 2 Gas separation properties of membranes in this work and model predictions
| Membrane | P/Barrer | Selectivity | |||||
|---|---|---|---|---|---|---|---|
| O2 | N2 | CH4 | CO2 | O2/N2 | CO2/CH4 | ||
| ODPA-TMPDA | 8.93 | 1.74 | 1.63 | 47.76 | 5.13 | 29.30 | |
| Cu3(BTC)2/ODPA-TMPDA | 35.47 | 7.07 | 7.00 | 180.80 | 5.02 | 26.64 | |
| S-Cu3(BTC)2/ODPA-TMPDA | 25.00 | 5.27 | 4.94 | 131.70 | 4.93 | 26.30 | |
| ZIF-8/ODPA-TMPDA | 38.79 | 8.90 | 7.71 | 189.80 | 4.81 | 23.53 | |
| Maxwell model | 20.41 | 3.98 | 3.73 | 109.20 | 5.13 | 29.30 | |
| Bruggeman model | 26.03 | 5.07 | 4.75 | 139.20 | 5.13 | 29.30 | |
| Membrane | 108 Diffusivity/(cm2·s-1) | Solubility/(cm3·g-1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| O2 | N2 | CH4 | CO2 | O2 | N2 | CH4 | CO2 | ||
| ODPA-TMPDA | 5.10 | 1.24 | 0.276 | 1.78 | 0.0176 | 0.0141 | 0.0591 | 0.269 | |
| Cu3(BTC)2/ODPA-TMPDA | 10.00 | 2.39 | 0.643 | 4.88 | 0.0359 | 0.0298 | 0.1080 | 0.371 | |
| S-Cu3(BTC)2/ODPA-TMPDA | 9.72 | 2.48 | 0.582 | 4.28 | 0.0267 | 0.0229 | 0.0839 | 0.310 | |
| ZIF-8/ODPA-TMPDA | 17.10 | 3.99 | 1.150 | 7.42 | 0.0227 | 0.0203 | 0.0669 | 0.256 | |
Table 3 Gas diffusivity and solubility of ODPA-TMPDA membrane and MOFs/ODPA-TMPDA mixed matrix membranes
| Membrane | 108 Diffusivity/(cm2·s-1) | Solubility/(cm3·g-1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| O2 | N2 | CH4 | CO2 | O2 | N2 | CH4 | CO2 | ||
| ODPA-TMPDA | 5.10 | 1.24 | 0.276 | 1.78 | 0.0176 | 0.0141 | 0.0591 | 0.269 | |
| Cu3(BTC)2/ODPA-TMPDA | 10.00 | 2.39 | 0.643 | 4.88 | 0.0359 | 0.0298 | 0.1080 | 0.371 | |
| S-Cu3(BTC)2/ODPA-TMPDA | 9.72 | 2.48 | 0.582 | 4.28 | 0.0267 | 0.0229 | 0.0839 | 0.310 | |
| ZIF-8/ODPA-TMPDA | 17.10 | 3.99 | 1.150 | 7.42 | 0.0227 | 0.0203 | 0.0669 | 0.256 | |
| [1] | Baker R. W., Industrial and Engineering Chemistry Research, 2002, 41(6), 1393—1411 |
| [2] | Hashim S. S., Mohamed A. R., Bhatia S., Renewable and Sustainable Energy Reviews, 2011, 15, 1284—1293 |
| [3] | Chen X., Wang Y., Jiang L., Chem. J. Chinese Universities, 2013, 34(2), 249—268 |
| (陈曦, 王耀, 江雷. 高等学校化学学报, 2013, 34(2), 249—268) | |
| [4] | Robeson L. M., Journal of Membrane Science, 2008, 320, 390—400 |
| [5] | Chung T. S., Jiang L. Y., Li Y., Kulprathipanja S., Progress in Polymer Science, 2007, 32(4), 483—507 |
| [6] | Nasir R., Mukhtar H., Man Z., Mohshim D. F., Chemical Engineering & Technology, 2013, 36(5), 717—727 |
| [7] | Xu D., Wang Y., Zhang Y., Zhang G., Shao K., Li S. W., Na H., Chem. Res. Chinese Universities, 2010, 26(6), 1031—1034 |
| [8] | Li J. R., Kuppler R. J., Zhou H. C., Chemical Society Reviews, 2009, 38, 1477—1504 |
| [9] | Na L. Y., Hua R. N.,, Ning G. L., Ou X. X., Chem. Res. Chinese Universities, 2012, 28(4), 555—558 |
| [10] | Zornoza B., Tellez C., Coronas J., Gascon J., Kapteijn F., Microporous and Mesoporous Materials, 2013, 166, 67—78 |
| [11] | Zhang Y. F., Musselman I. H., Ferraris J. P., Balkus K. J., Journal of Membrane Science, 2008, 313, 170—181 |
| [12] | Perez E. V., Balkus K. J., Ferraris J. P., Musselman I. H., Journal of Membrane Science, 2009, 328, 165—173 |
| [13] | Fletcher A. J., Thomas K. M., Rosseinsky M. J., Journal of Solid State Chemistry, 2005, 178, 2491—2510 |
| [14] | Tantekin-Ersolmaz S. B., Atalay-Orala C., Tather M., Erdem-Senatalar A., Schoeman B., Sterte J., Journal of Membrane Science, 2000, 175, 285—288 |
| [15] | Liu J. C., Culp J. T., Natesakhawat S., Journal of Physical Chemistry C, 2007, 111, 9305—9313 |
| [16] | Zhuang J. L., Ceglarek D., Pethuraj S., Terfort A., Advanced Functional Materials, 2011, 21, 1442—1447 |
| [17] | Cravillon J., Muenzer S., Lohmeier S. J., Chemistry of Materials, 2009, 21(8), 1410—1412 |
| [18] | Qiu X. Z., Cao Y. M., Wang L. N., Zhou M. Q, Yuan Q., Chem. J. Chinese Universities, 2009, 30(1), 196—202 |
| (邱晓智, 曹义鸣, 王丽娜, 周美青, 袁权. 高等学校化学学报, 2009, 30(1), 196—202) | |
| [19] | Duan C. |
| [20] | Wang L. N., Cao Y. M., Zhou M. Q., Journal of Membrane Science, 2007, 305, 338—346 |
| [21] | Basu S., Cano-Odena A., Vankelecom I. J., Journal of Membrane Science, 2010, 362, 478—487 |
| [22] | Kim S., Marand E., Chemistry of Materials, 2006, 18(5), 1149—1155 |
| (Ed.: W, Z) |
| [1] | QIU Xinsheng, WU Qin, SHI Daxin, ZHANG Yaoyuan, CHEN Kangcheng, LI Hansheng. Preparation and High Temperature Fuel Cell Performance of Ionic Crosslinked Sulfonated Polyimides for Proton Exchange Membranes [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220140. |
| [2] | ZHAO Yingzhe, ZHANG Jianling. Applications of Metal-organic Framework-based Material in Carbon Dioxide Photocatalytic Conversion [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220223. |
| [3] | LU Cong, LI Zhenhua, LIU Jinlu, HUA Jia, LI Guanghua, SHI Zhan, FENG Shouhua. Synthesis, Structure and Fluorescence Detection Properties of a New Lanthanide Metal-Organic Framework Material [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220037. |
| [4] | ZHAO Junyu, WANG Chunbo, WANG Chengyang, ZHANG Ke, CONG Bing, YANG Lan, ZHAO Xiaogang, CHEN Chunhai. Preparation and Performance of Thermally Conductive Expanded Graphite/Polyetherimide Composites [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210800. |
| [5] | TIAN Xueqin, MO Zheng, DING Xin, WU Pengyan, WANG Yu, WANG Jian. A Squaramide-containing Luminescent Metal-organic Framework as a High Selective Sensor for Histidine [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210589. |
| [6] | WANG Shoubai, WU Xiuming, SHU Chen, ZHONG Min, HUANG Wei, YAN Deyue. Gas Separation Performance of Polyimide Homogeneous MembranesContaining tert-Butyl Groups [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220357. |
| [7] | XING Peiqi, LU Tong, LI Guanghua, WANG Liyan. Controllable Syntheses of Two Cd(II) Metal-organic Frameworks Possessing Related Structures [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220218. |
| [8] | LI Shurong, WANG Lin, CHEN Yuzhen, JIANG Hailong. Research Progress of Metal⁃organic Frameworks on Liquid Phase Catalytic Chemical Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210575. |
| [9] | ZHANG Chi, SUN Fuxing, ZHU Guangshan. Synthesis, N2 Adsorption and Mixed-matrix Membrane Performance of Bimetal Isostructural CAU-21 [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210578. |
| [10] | MO Zongwen, ZHANG Xuewen, ZHOU Haolong, ZHOU Dongdong, ZHANG Jiepeng. Guest-responses of A Porous Coordination Polymer Based on Synergistic Hydrogen Bonds [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210576. |
| [11] | LIU Xueguang, YANG Xiaoshan, MA Jingjing, LIU Weisheng. Separating Methyl Blue Selectively from the Mixture of Dyes by Europium Metal-organic Frameworks [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210715. |
| [12] | HAN Zongsu, YU Xiaoyong, MIN Hui, SHI Wei, CHENG Peng. A Rare Earth Metal-Organic Framework with H6TTAB Ligand [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210342. |
| [13] | SHI Xiaofan, ZHU Jian, BAI Tianyu, FU Zixuan, ZHANG Jijie, BU Xianhe. Research Status and Progress of MOFs with Application in Photoelectrochemical Water-splitting [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210613. |
| [14] | WU Ji, ZHANG Hao, LUO Yuhui, GENG Wuyue, LAN Yaqian. A Microporous Cationic Ga(III)-MOF with Fluorescence Properties for Selective sensing Fe3+ Ion and Nitroaromatic Compounds [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210617. |
| [15] | LI Wen, QIAO Junyi, LIU Xinyao, LIU Yunling. Zirconium-based Metal-Organic Framework with Naphthalene for Fluorescent Detection of Nitroaromatic Explosives in Water [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210654. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||