

Chem. J. Chinese Universities ›› 2021, Vol. 42 ›› Issue (1): 227.doi: 10.7503/cjcu20200413
Special Issue: 分子筛功能材料 2021年,42卷,第1期
• Article • Previous Articles Next Articles
GUO Shujia1,2, WANG Sen1( ), ZHANG Li1,2, QIN Zhangfeng1(
), ZHANG Li1,2, QIN Zhangfeng1( ), WANG Pengfei1, DONG Mei1, WANG Jianguo1,2, FAN Weibin1(
), WANG Pengfei1, DONG Mei1, WANG Jianguo1,2, FAN Weibin1( )
)
Received:2020-07-01
Online:2021-01-10
Published:2021-01-12
Contact:
WANG Sen,QIN Zhangfeng,FAN Weibin
E-mail:wangsen@sxicc.ac.cn;qzhf@sxicc.ac.cn;fanwb@sxicc.ac.cn
Supported by:CLC Number:
TrendMD:
GUO Shujia, WANG Sen, ZHANG Li, QIN Zhangfeng, WANG Pengfei, DONG Mei, WANG Jianguo, FAN Weibin. Regulating the Acid Sites Distribution in ZSM-5 Zeolite and Its Catalytic Performance in the Conversion of Methanol to Olefins[J]. Chem. J. Chinese Universities, 2021, 42(1): 227.
| Zeolite | Crystallinity (%) | n(Si)/n(Al) | Surface area/(m2·g-1) | Pore volume/(cm3·g-1) | ||
|---|---|---|---|---|---|---|
| Total | Micro | Total | Micro | |||
| ZSM-5-0Na | 100 | 36.1 | 430.2 | 294.1 | 0.37 | 0.11 |
| ZSM-5-0.2Na | 93.5 | 35.0 | 364.6 | 275.6 | 0.24 | 0.12 |
| ZSM-5-0.4Na | 92.9 | 35.6 | 399.5 | 312.6 | 0.25 | 0.10 |
| ZSM-5-0.6Na | 92.4 | 33.5 | 351.4 | 277.0 | 0.22 | 0.08 |
Table 1 Chemical composition and textural properties of all ZSM-5 zeolites*
| Zeolite | Crystallinity (%) | n(Si)/n(Al) | Surface area/(m2·g-1) | Pore volume/(cm3·g-1) | ||
|---|---|---|---|---|---|---|
| Total | Micro | Total | Micro | |||
| ZSM-5-0Na | 100 | 36.1 | 430.2 | 294.1 | 0.37 | 0.11 |
| ZSM-5-0.2Na | 93.5 | 35.0 | 364.6 | 275.6 | 0.24 | 0.12 |
| ZSM-5-0.4Na | 92.9 | 35.6 | 399.5 | 312.6 | 0.25 | 0.10 |
| ZSM-5-0.6Na | 92.4 | 33.5 | 351.4 | 277.0 | 0.22 | 0.08 |
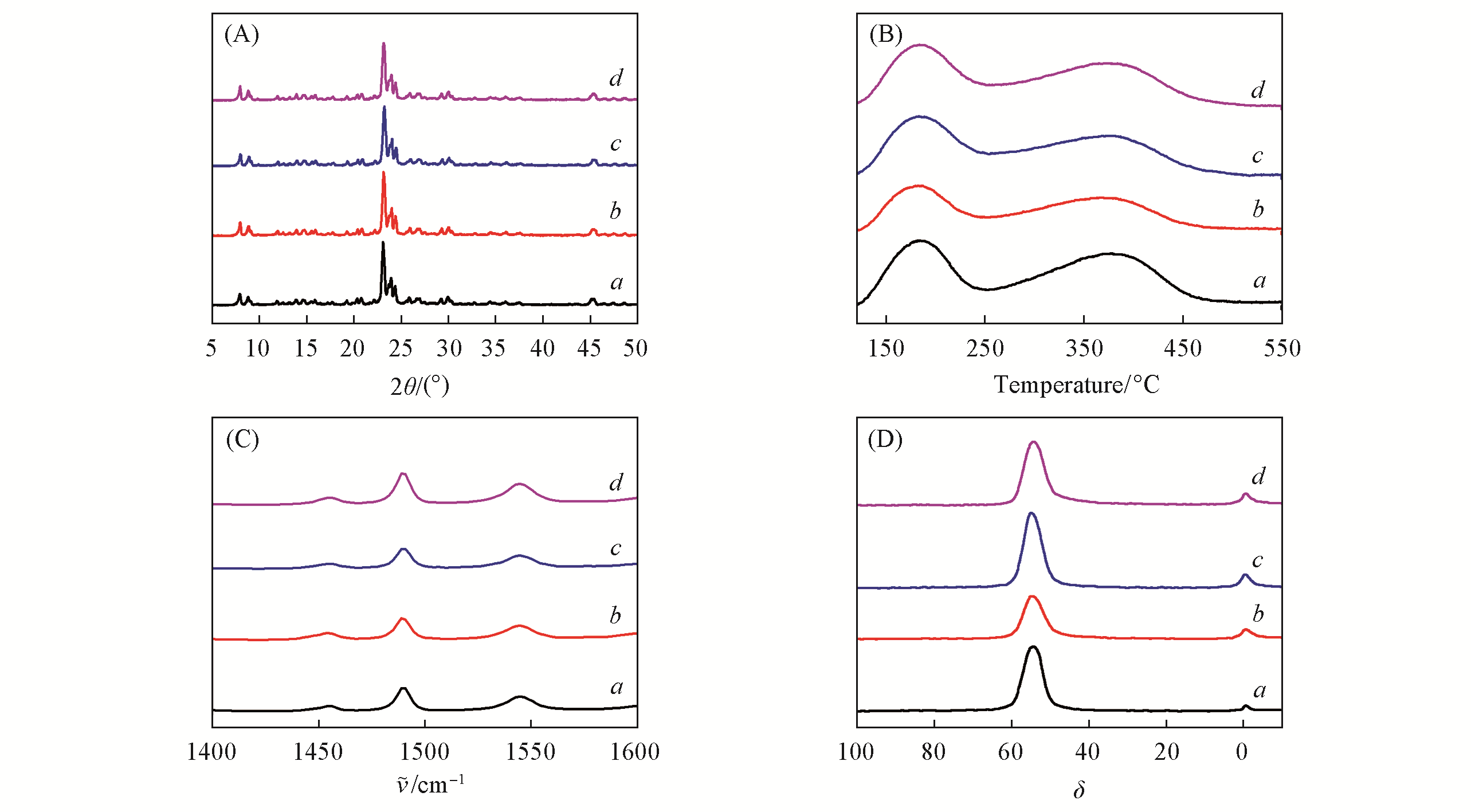
Fig.1 XRD patterns(A), NH3?TPD profiles(B), Py?IR spectra collected at 423 K(C) and 27Al solid?state MAS NMR spectra(D) of ZSM?5?0Na(a), ZSM?5?0.2Na(b), ZSM?5?0.4Na(c) and ZSM?5?0.6Na(d)
| Zeolite | Acidity(423 K)/(μmol·g-1) | Acidity(523 K)/(μmol·g-1) | Acidity(623 K)/(μmol·g-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Br?nsted | Lewis | Total | Br?nsted | Lewis | Total | Br?nsted | Lewis | |
| ZSM?5?0Na | 220.0 | 178.4 | 41.6 | 186.0 | 158.5 | 27.5 | 154.5 | 132.3 | 22.2 |
| ZSM?5?0.2Na | 206.4 | 150.9 | 55.5 | 158.5 | 131.3 | 27.2 | 129.0 | 105.6 | 23.4 |
| ZSM?5?0.4Na | 204.0 | 162.6 | 41.4 | 160.5 | 135.9 | 24.6 | 132.7 | 113.1 | 19.6 |
| ZSM?5?0.6Na | 241.1 | 200.3 | 40.8 | 201.3 | 169.9 | 31.4 | 158.9 | 133.9 | 25.0 |
Table 2 Acid property of different ZSM-5 zeolites determined by Py-IR*
| Zeolite | Acidity(423 K)/(μmol·g-1) | Acidity(523 K)/(μmol·g-1) | Acidity(623 K)/(μmol·g-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Br?nsted | Lewis | Total | Br?nsted | Lewis | Total | Br?nsted | Lewis | |
| ZSM?5?0Na | 220.0 | 178.4 | 41.6 | 186.0 | 158.5 | 27.5 | 154.5 | 132.3 | 22.2 |
| ZSM?5?0.2Na | 206.4 | 150.9 | 55.5 | 158.5 | 131.3 | 27.2 | 129.0 | 105.6 | 23.4 |
| ZSM?5?0.4Na | 204.0 | 162.6 | 41.4 | 160.5 | 135.9 | 24.6 | 132.7 | 113.1 | 19.6 |
| ZSM?5?0.6Na | 241.1 | 200.3 | 40.8 | 201.3 | 169.9 | 31.4 | 158.9 | 133.9 | 25.0 |
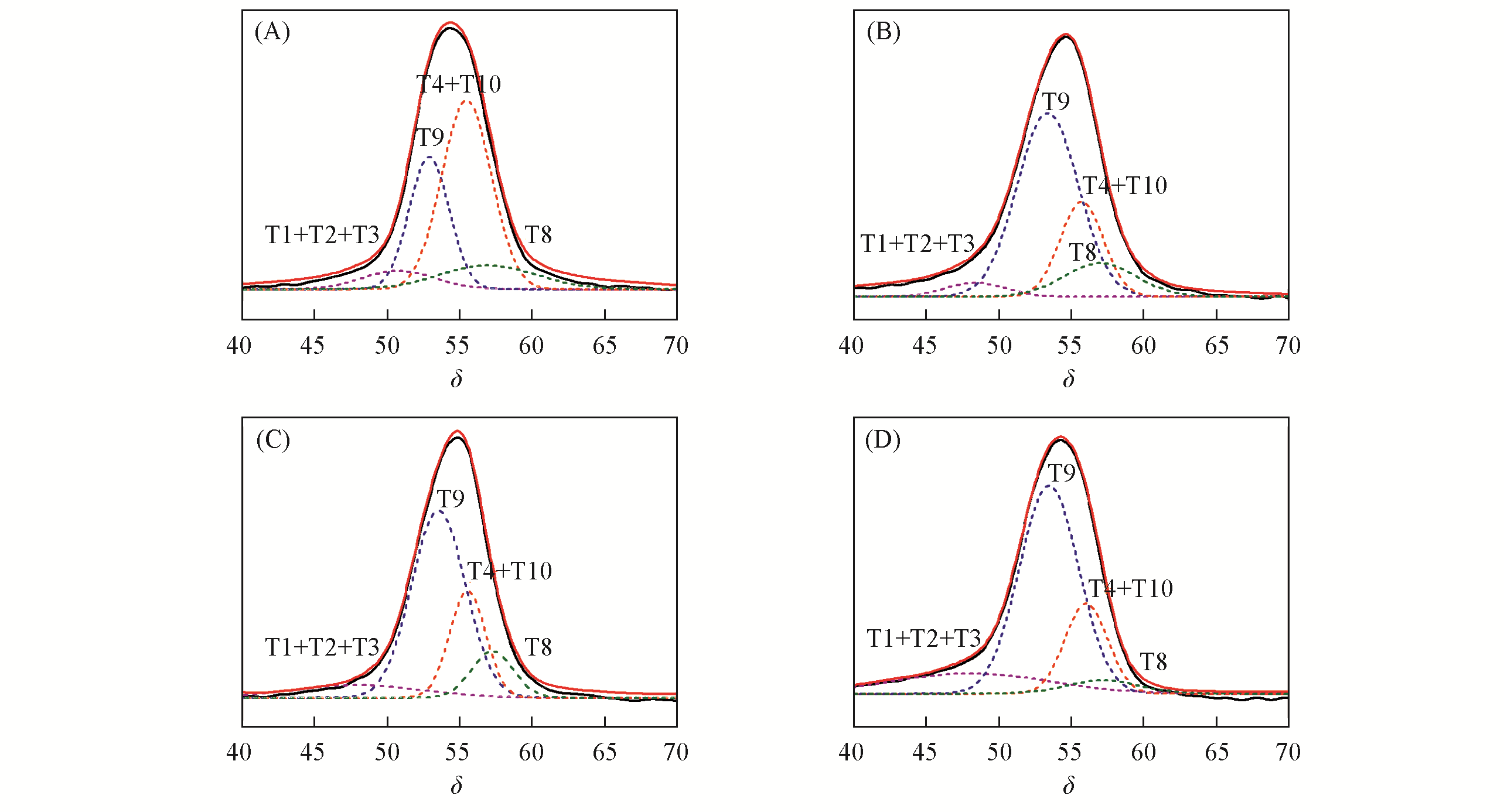
Fig.2 Deconvolution of the 27Al MAS NMR spectra of ZSM?5?0Na(A), ZSM?5?0.2Na(B), ZSM?5?0.4Na(C) and ZSM?5?0.6Na(D)The experimental spectra are shown in black lines and the fitted ones in red lines. The Al atoms at different T sites were assigned by referring the chemical shifts estimated by the DFT computation[11].
| Zeolite | AlEF(%) | AlF(%) | Al distribution(%) | |||
|---|---|---|---|---|---|---|
| δ 50.0 | δ 53.2 | δ 56.5 | δ 57.9 | |||
| ZSM?5?0Na | 2.4 | 97.6 | 7.6 | 28.7 | 50.7 | 13.0 |
| ZSM?5?0.2Na | 9.0 | 91.0 | 4.4 | 60.6 | 22.1 | 12.9 |
| ZSM?5?0.4Na | 8.2 | 91.8 | 9.4 | 57.5 | 22.0 | 11.1 |
| ZSM?5?0.6Na | 5.7 | 94.3 | 16.0 | 60.2 | 18.8 | 5.0 |
Table 3 Proportion of integrated peak area obtained by the deconvolution of 27Al MAS NMR spectra of ZSM-5 zeolites*
| Zeolite | AlEF(%) | AlF(%) | Al distribution(%) | |||
|---|---|---|---|---|---|---|
| δ 50.0 | δ 53.2 | δ 56.5 | δ 57.9 | |||
| ZSM?5?0Na | 2.4 | 97.6 | 7.6 | 28.7 | 50.7 | 13.0 |
| ZSM?5?0.2Na | 9.0 | 91.0 | 4.4 | 60.6 | 22.1 | 12.9 |
| ZSM?5?0.4Na | 8.2 | 91.8 | 9.4 | 57.5 | 22.0 | 11.1 |
| ZSM?5?0.6Na | 5.7 | 94.3 | 16.0 | 60.2 | 18.8 | 5.0 |
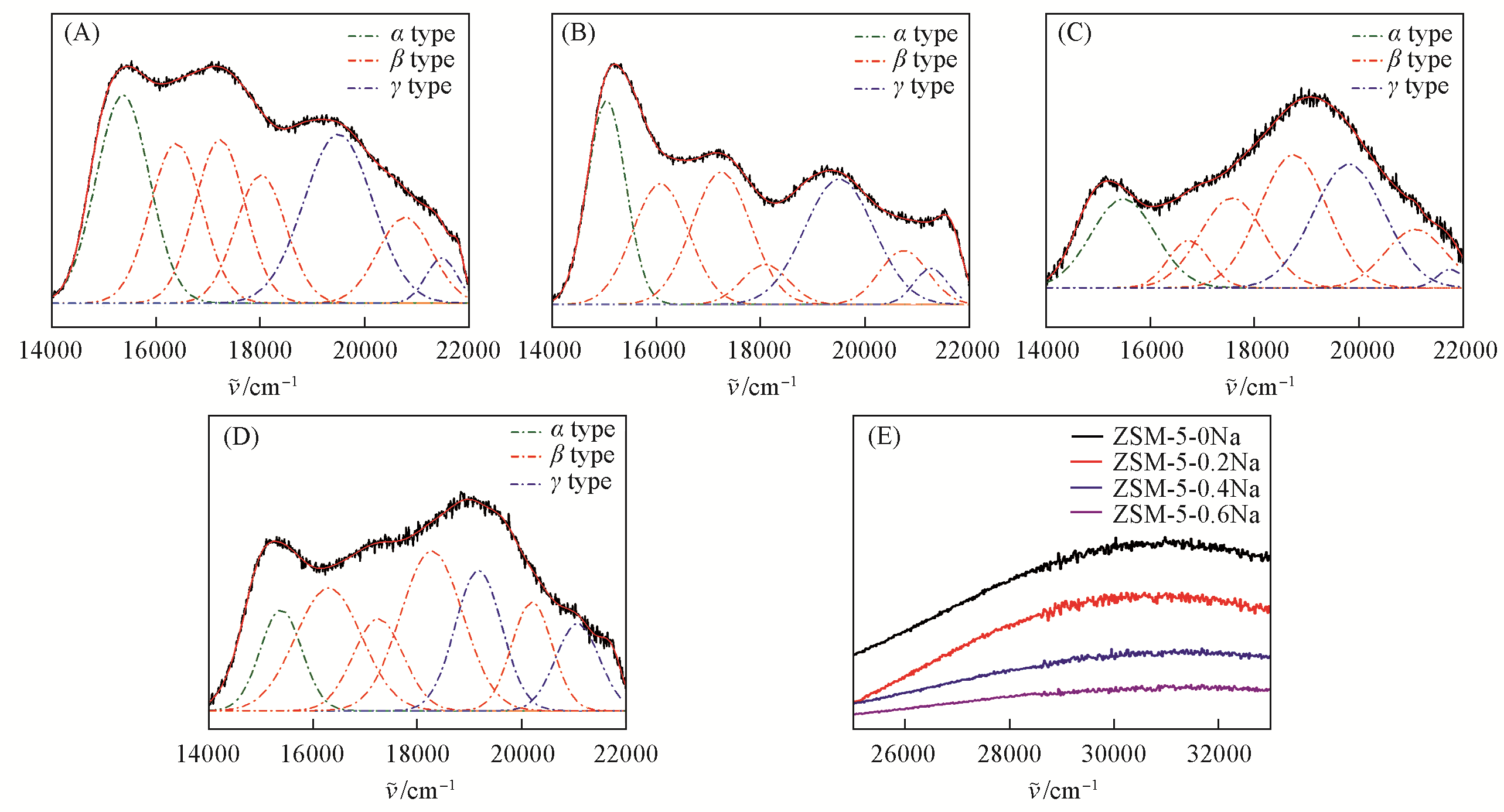
Fig.3 Deconvolution of the DR UV?Vis spectra of ZSM?5?0Na(A), ZSM?5?0.2Na(B), ZSM?5?0.4Na(C) and ZSM?5?0.6Na(D) and the region of 25000—33000 cm-1 of various Co2+?exchanged ZSM?5 zeolites(E)The experimental spectra are shown in black lines and the fitted ones in red lines.
| Zeolite | n(Si)/n(Al) | Alpairs(%) | Alsingle(%) | Al distribution(%) | ||
|---|---|---|---|---|---|---|
| α type | β type | γ type | ||||
| ZSM?5?0Na | 37.2 | 75.4 | 24.6 | 26.4 | 43.3 | 30.3 |
| ZSM?5?0.2Na | 35.9 | 83.1 | 16.9 | 21.1 | 58.6 | 20.3 |
| ZSM?5?0.4Na | 35.5 | 72.2 | 37.8 | 16.3 | 62.3 | 21.4 |
| ZSM?5?0.6Na | 37.1 | 80.0 | 20.0 | 14.0 | 77.0 | 9.0 |
Table 4 Distributions of different Al species in the ZSM-5 zeolite as measured by DR UV-Vis spectra of Co2+-exchanged ZSM-5 zeolites*
| Zeolite | n(Si)/n(Al) | Alpairs(%) | Alsingle(%) | Al distribution(%) | ||
|---|---|---|---|---|---|---|
| α type | β type | γ type | ||||
| ZSM?5?0Na | 37.2 | 75.4 | 24.6 | 26.4 | 43.3 | 30.3 |
| ZSM?5?0.2Na | 35.9 | 83.1 | 16.9 | 21.1 | 58.6 | 20.3 |
| ZSM?5?0.4Na | 35.5 | 72.2 | 37.8 | 16.3 | 62.3 | 21.4 |
| ZSM?5?0.6Na | 37.1 | 80.0 | 20.0 | 14.0 | 77.0 | 9.0 |
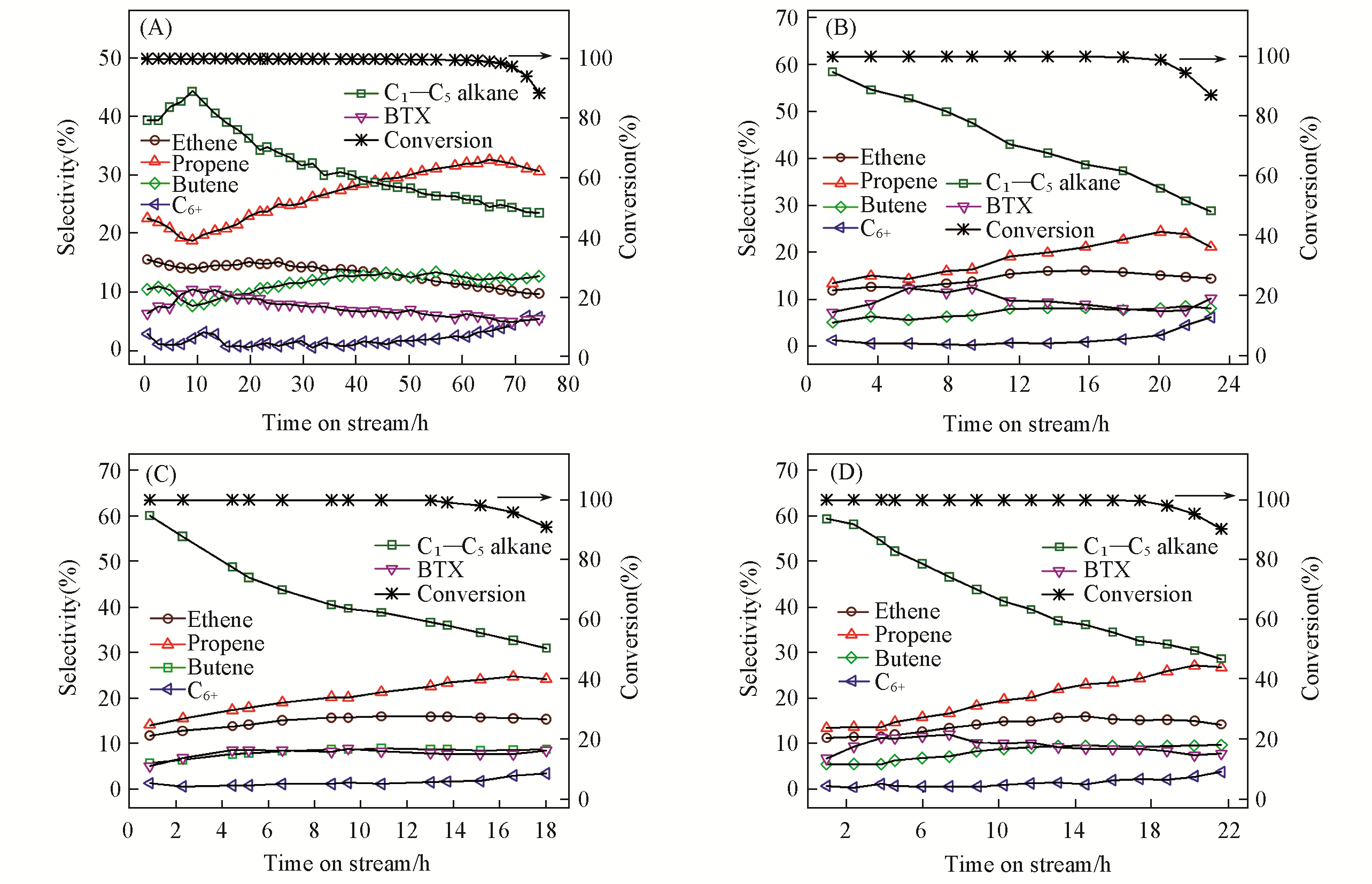
Fig.4 Variation of the methanol conversion and product selectivity with time on stream for MTO over the ZSM?5?0Na(A), ZSM?5?0.2Na(B), ZSM?5?0.4Na(C) and ZSM?5?0.6Na(D) zeolites under atmospheric pressure and 723 K, with a methanol WHSV of 3.8 h-1
| Zeolite | Conv. (%) | Product selectivity(%) | HTI | Lifetime /h | 10?4TON | (P-E)/E | 2MB/E | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | C | C | C1—C 5 | BTX | C4-HTI | C5-HTI | ||||||
| ZSM?5?0Na | 99.8 | 13.8 | 29.1 | 20.0 | 30.3 | 6.8 | 0.48 | 0.55 | 74.4 | 4.92 | 1.11 | 1.00 |
| ZSM?5?0.2Na | 99.9 | 15.5 | 19.2 | 12.6 | 43.0 | 9.7 | 0.69 | 0.67 | 22.9 | 1.77 | 0.23 | 0.67 |
| ZSM?5?0.4Na | 99.9 | 15.6 | 20.0 | 15.1 | 39.6 | 8.8 | 0.66 | 0.63 | 18.0 | 1.30 | 0.28 | 0.87 |
| ZSM?5?0.6Na | 99.9 | 14.8 | 19.6 | 14.2 | 41.5 | 9.9 | 0.67 | 0.63 | 21.6 | 1.27 | 0.32 | 0.82 |
Table 5 Catalytic test results for MTO over the ZSM-5 zeolites with different acid sites distributions*
| Zeolite | Conv. (%) | Product selectivity(%) | HTI | Lifetime /h | 10?4TON | (P-E)/E | 2MB/E | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | C | C | C1—C 5 | BTX | C4-HTI | C5-HTI | ||||||
| ZSM?5?0Na | 99.8 | 13.8 | 29.1 | 20.0 | 30.3 | 6.8 | 0.48 | 0.55 | 74.4 | 4.92 | 1.11 | 1.00 |
| ZSM?5?0.2Na | 99.9 | 15.5 | 19.2 | 12.6 | 43.0 | 9.7 | 0.69 | 0.67 | 22.9 | 1.77 | 0.23 | 0.67 |
| ZSM?5?0.4Na | 99.9 | 15.6 | 20.0 | 15.1 | 39.6 | 8.8 | 0.66 | 0.63 | 18.0 | 1.30 | 0.28 | 0.87 |
| ZSM?5?0.6Na | 99.9 | 14.8 | 19.6 | 14.2 | 41.5 | 9.9 | 0.67 | 0.63 | 21.6 | 1.27 | 0.32 | 0.82 |
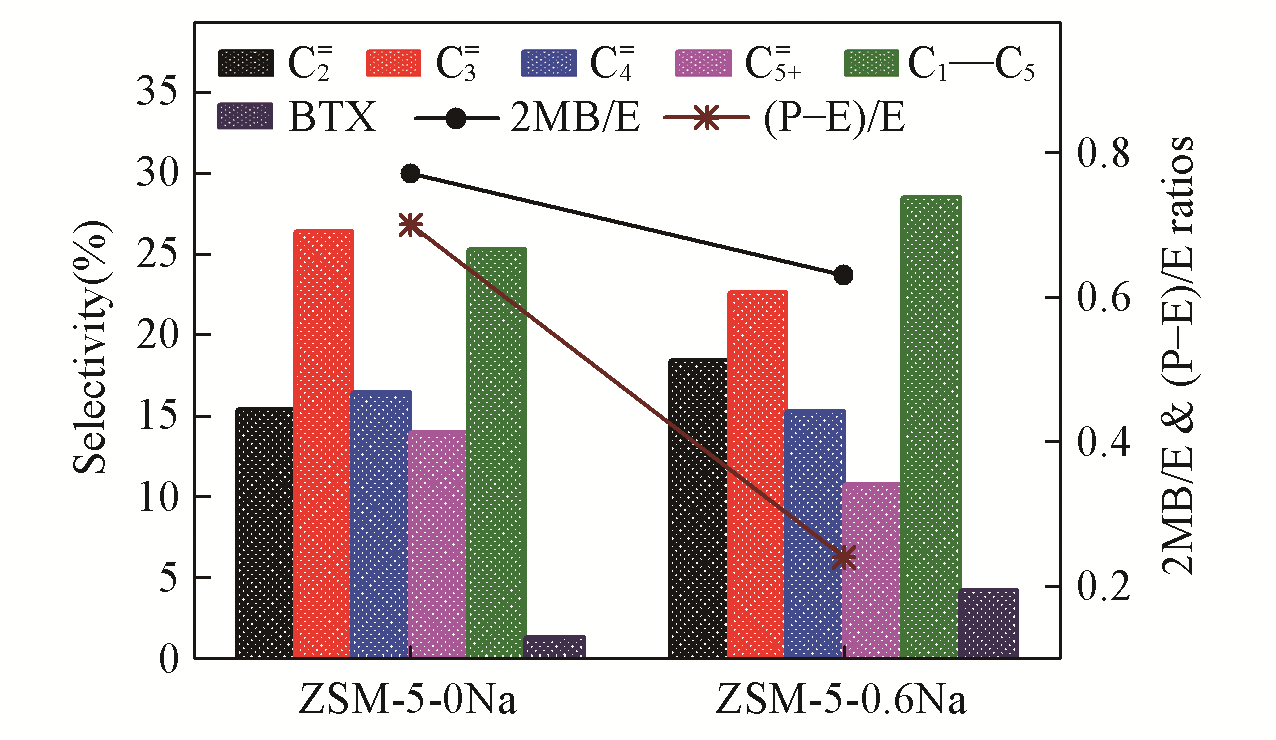
Fig.5 A comparison in the selectivities to ethene(C2???=), propene(C3???=), butene(C4???=), alkenes higher than butene(C5+=), C1―C5 alkanes, and benzene, toluene and xylenes(BTX) as well as the (P-E)/E and 2MB/E(P for propene, E for ethene, and 2MB for 2?methylbutane and 2?methyl?2?butene) for MTO over the ZSM?5?0Na and ZSM?5?0.6Na under atmospheric pressure and 623 K, with a WHSV of 48 h-1 and a methanol conversion of 75%, reported at 30 min on stream
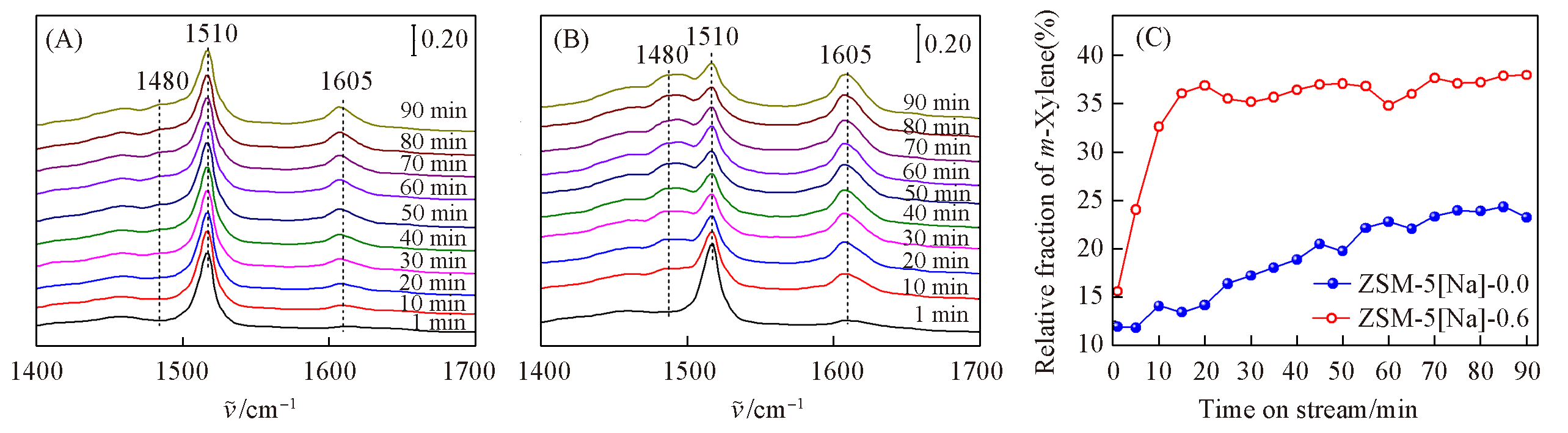
Fig.6 Time?resolved IR spectra of p?xylene isomerization over ZSM?5?0Na(A) and ZSM?5?0.6Na(B) at 473 K as well as the relative fraction of m?xylene(C)(C) Obtained with the peak area of m?xylene divided by the total peak areas of p?, o?, and m?xylenes in situ IR spectra. p?Xylene with a partial pressure of 300 Pa was continuously introduced into the reaction cell and the pressure was kept constant during the whole reaction process.
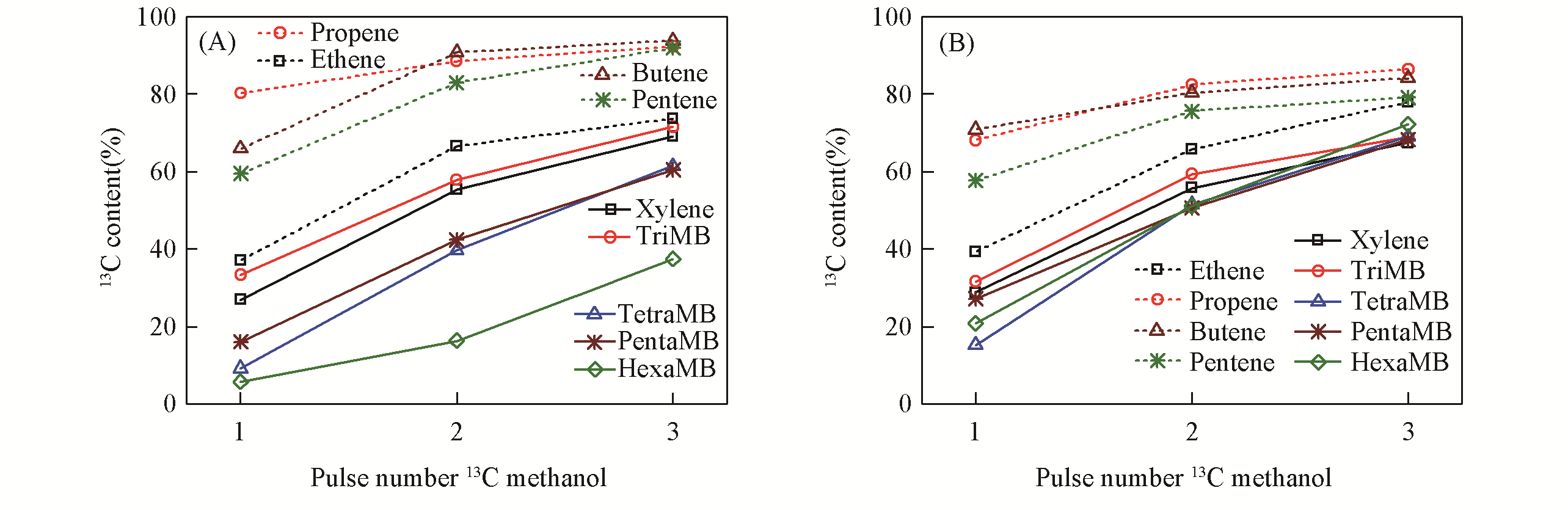
Fig.9 13C contents in the effluent olefins(ethene to pentene) and in the retained aromatics(polyMBs) for MTO over ZSM?5?0Na(A) and ZSM?5?0.6Na(B) zeolites at 553 K
| 1 | Haw J., Song W., Marcus D., Nicholas J., Acc. Chem. Res., 2003, 36, 317—326 |
| 2 | Olsbye U., Svelle S., Bjørgen M., Beato P., Janssens T. V. W., Joensen F., Bordiga S., Lillerud K. P., Angew. Chem. Int. Ed., 2012, 51(24), 5810—5831 |
| 3 | Tian P., Wei Y., Ye M., Liu Z., ACS Catal., 2015, 5(3), 1922—1938 |
| 4 | Xu S., Zhi Y., Han J., Zhang W., Wu X., Sun T., Wei Y., Liu Z., Adv Catal., 2017, 61, 37—122 |
| 5 | Wang C., Chu Y., Zheng A., Xu J., Wang Q., Gao P., Qi G., Gong Y., Deng F., Chem. Eur. J., 2014, 20(39), 1—13 |
| 6 | Dai W., Wan C., Dyballa M., Wu G., Guan N., Li L., Xie Z., Hunger M., ACS Catal., 2015, 5(1), 317—326 |
| 7 | Dedecek J., Sobalik Z., Wichterlova B., Catal. Rev. Sci. Eng., 2012, 54, 135—223 |
| 8 | Knott B., Nimlos C., Robichaud D., Nimlos M., Kim S., Gounder R., ACS Catal., 2018, 8(2),770—784 |
| 9 | Wang S., He Y., Jiao W., Wang J., Fan W., Curr. Opin. Chem. Eng., 2019,23, 146—154 |
| 10 | Chen J., Liang T., Li J., Wang S., Qin Z., Wang P., Huang L., Fan W., Wang J., ACS Catal., 2016, 6(4), 2299—2313 |
| 11 | Wang S., Wang P., Qin Z., Chen Y., Dong M., Li J., Zhang K., Liu P., Wang J., Fan W., ACS Catal., 2018, 8(6), 5485—5505 |
| 12 | Liang T., Chen J., Qin Z., Li J., Wang P., Wang S., Wang G., Dong M., Fan W., Wang J., ACS Catal., 2016, 6(11), 7311—7325 |
| 13 | Dedecek J., Balgova V., Pashkova V., Klein P., Wichterlova B., Chem. Mater., 2012, 24(16), 3231—3239 |
| 14 | Yokoi T., Mochizuki H., Namba S., Kondo J. N., Tatsumi T., J. Phys. Chem. C, 2015, 119(27), 15303—15315 |
| 15 | Pashkovava V., Sklenak S., Klein P., Urbanova M., Dedecek J., Chem. Eur. J., 2016, 22(12), 3937 —3941 |
| 16 | Di Iorio J., Gounder R., Chem. Mater., 2016, 28(7),2236—2247 |
| 17 | Zhao X., Wang L., Li J., Xu S., Zhang W., Wei Y., Guo X., Tian P., Liu Z., Catal. Sci. Technol., 2017, 7(4), 5882—5892 |
| 18 | Sazama P., Dedecek J., Gabova V., Wichterlova B., Spoto G., Bordiga S., J. Catal., 2008, 254(2), 180—189 |
| 19 | Wang S., Zhang L., Li S., Qin Z., Shi D., He S., Yuan K., Wang P., Zhao T., Fan S., Dong M., Li J., Fan W., J. Catal., 2019, 377, 81—97 |
| 20 | Price G., Iglesia E., Ind. Eng. Chem. Res., 1989, 28(6), 839—844 |
| 21 | Vjunov A., Fulton J. L., Huthwelker T., Pin S., Mei D. H., Schenter G. K., Govind N., Camaioni D. M., Hu J. Z., Lercher J. A., J. Am. Chem. Soc., 2014, 136(23), 8296-8306 |
| 22 | Liu Z., Dong X., Zhu Y., Emwas A., Zhang D., Tian Q., Han Y., ACS Catal., 2015, 5(10), 5837—5845 |
| 23 | Dedecek J., Lucero M. J., Li C. B., Gao F., Klein P., Urbanova M., Tvaruzkova Z., Sazama P., Sklenak S., J. Phys. Chem. C, 2011, 115(22), 11056—11064 |
| 24 | Sklenak S., Dedecek J., Li C. B., Wichterlova B., Gabova V., Sierka M., Sauer J., Angew. Chem. Int. Ed., 2007, 46(38), 7286—7289 |
| 25 | Zhao R., Zhao Z., Li S., Zhang W., J. Phys. Chem. Lett., 2017, 8(10), 2323—2327 |
| 26 | Pashkovava V., Sklenak S., Klein P., Urbanova M., Dedecek J., Chem. Eur. J., 2016, 22(12), 3937—3941 |
| 27 | Ilias S., Khare R., Malek A., Bhan A., J. Catal., 2013, 303, 135—140 |
| 28 | Bleken F., Janssens T. V. W., Svelle S., Olsbye U., Microporous Mesoporous Mater., 2012, 164, 190—198 |
| 29 | Shen Y., Le T., Fu D., Schmidt J., Filez M., Weckhuysen B., Rimer J., ACS Catal., 2018, 8(12), 11042—11053 |
| 30 | Gomez⁃Hortiguela L., Pinar A., Cora F., Perea⁃Pariente J., Chem. Commun., 2010, 46(12), 2073—2075 |
| 31 | Pinar A., Marquez⁃Alvarez C., Grande⁃Casas M., Perez⁃Pariente J., J. Catal., 2009, 263(2), 258—265 |
| 32 | Zheng S., Jentys A., Lercher J. A., J. Catal., 2006, 241(2), 304—311 |
| 33 | Bjørgen M., Bonino F., Kolboe S., Lillerud K., Zecchina A. Bordiga S., J. Am. Chem. Soc., 2003, 125(51), 15863—15868 |
| 34 | Dai W., Wu G., Li L., Guan N., Hunger M., ACS Catal., 2013, 3(4), 588—596 |
| 35 | Borodina E., Meirer F., Lezcano⁃Gonzalez I., Mokhtar M., Asiri A., Al⁃Thabaiti S., Basahel S., Ruiz⁃Martinez J., Weckhuysen B., ACS Catal., 2015, 5(2), 992—1003 |
| 36 | Goetze J., Meirer F., Yarulina I., Gascon J., Kapteijn F., Ruiz⁃Martinez J., Weckhuysen B., ACS Catal., 2017, 7(6), 4033—4046 |
| 37 | Van Speybroeck V., Hemelsoet K., De Wispelaere K., Qian Q., Van der Mynsbrugge J., De Sterck B., Weckhuysen B., Waroquier M., ChemCatChem, 2013, 5(1), 173—184 |
| 38 | Wang S., Chen Y., Qin Z., Zhao T., Fan S., Dong M., Li J., Fan W., Wang J., J. Catal., 2019, 369, 382—395 |
| 39 | Xu T., Barich D., Goguen P., Song W., Wang Z., Nicholas J., Haw J., J. Am. Chem. Soc., 1998, 120(16), 4025—4026 |
| 40 | Haw J., Nicholas J., Song W., Deng F., Wang Z., Xu T., Heneghan C., J. Am. Chem. Soc., 2000, 122(19), 4763—4775 |
| 41 | Li J., Wei Y., Chen J., Tian P., Su X., Xu S., Qi Y., Wang Q., Zhou Y., He Y., Liu Z., J. Am. Chem. Soc., 2012, 134(2), 836— 839 |
| 42 | Wang C., Xu J., Qi G., Gong Y., Wang W., Gao P., Wang Q., Feng N., Liu X., Deng F., J. Catal., 2015, 332, 127—137 |
| 43 | Zhang W., Chen J., Xu S., Chu Y., Wei Y., Zhi Y., Huang J., Zhang A., Wu X., Meng X., Xiao F., Deng F., Liu Z., ACS Catal., 2018, 8(12), 10950—10963 |
| [1] | PENG Haiyue, WANG Ting, LI Guorui, HUANG Jing. Synthesis of Melanin and Its Function Regulation by Small Molecules [J]. Chem. J. Chinese Universities, 2021, 42(11): 3357. |
| [2] | LIN Ningqin, YAO Ke, CHEN Xiangjun. Research Progress of Molecular Recognition and Interaction of Crystallins Linking Cataract [J]. Chem. J. Chinese Universities, 2021, 42(11): 3379. |
| [3] | HUANG Ling, ZHUANG Zijian, LI Xiang, SHI Muling, LIU Gaoqiang. Advances in Molecular Recognition of Exosomes Based on Aptamers [J]. Chem. J. Chinese Universities, 2021, 42(11): 3493. |
| [4] | WANG Qing, HE Yuqiu, WANG Fuan. Advances of Multifunctional Deoxyribozyme in Biomedical Analysis [J]. Chem. J. Chinese Universities, 2021, 42(11): 3334. |
| [5] | LIU Xuejiao, YANG Fan, LIU Shuang, ZHANG Chunjuan, LIU Qiaoling. Progress in Aptamer-targeted Membrane Protein Recognition and Functional Regulation [J]. Chem. J. Chinese Universities, 2021, 42(11): 3277. |
| [6] | YU Xia, SONG Chenhai, GUO Xiangke, XUE Nianhua, DING Weiping. Cooperative Catalysis of Adjacent Acid Sites in Zeolite HZSM-5 [J]. Chem. J. Chinese Universities, 2021, 42(1): 239. |
| [7] | LI Jingying, CHEN Chen, LI Juan, YANG Huanghao. Artificial Regulation of Receptor Clustering and Function on Cell Surface † [J]. Chem. J. Chinese Universities, 2020, 41(5): 892. |
| [8] | WANG Xia, LIU Yanji, JIA Yongfeng, JI Lei, HU Quanli, DUAN Limei, LIU Jinghai. Preparative Chemistry of N-containing Porous Carbon Nanofibers for Capacity Improvement in Lithium-sulfur Battery † [J]. Chem. J. Chinese Universities, 2020, 41(4): 829. |
| [9] | WANG Wu, LAI Hua, CHENG Zhongjun, LIU Yuyan. Reversible Regulation of Droplet Directional/anti-directional Rolling on Superhydrophobic Shape Memory Microarray Surface [J]. Chem. J. Chinese Universities, 2020, 41(11): 2538. |
| [10] | WANG Lin, ZHANG Yanhui, Arzugul Muslim, LAN Haidie. Morphology and Size Regulation of Polyaniline Induced by PS-b-P2VP as Template and Its Electrochemical Characters [J]. Chem. J. Chinese Universities, 2019, 40(8): 1748. |
| [11] | ZHANG Wenli,NIE Yao,JING Xiaoran,XU Yan. Synthesis of 4-Hydroxyisoleucine Catalyzed by Recombinant Escherichia coli Expressing Fe(Ⅱ)/2-Ketoglutarate-dependent Dioxygenase† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1172. |
| [12] | BIAN Kai, HOU Zhanggui, DUAN Xinrui, LI Xiaoguo, CHANG Yang, CAO Hui, ZHANG Anfeng, GUO Xinwen. Synthesis and Catalytic Performance of 2D HZSM-5 Nano-sheet for Ethylbenzene Production from Benzene with Dilute Ethylene [J]. Chem. J. Chinese Universities, 2019, 40(4): 784. |
| [13] | ZHANG Zhang,WANG Dong,WANG Xiaolei,XU Yan. Regulation of Ester Synthesis Activity of Rhizopus chinensis Lipase† [J]. Chem. J. Chinese Universities, 2019, 40(4): 747. |
| [14] | Siqi SUN,Ying WANG,Chuanyin SUN,Runwei WANG,Zhendong ZHANG,Zongtao ZHANG,Shilun QIU. Preparation and Catalytic Performance of Bowl-shaped Amphiphilic ZSM-5 Zeolites Supported Gold Nanoparticles † [J]. Chem. J. Chinese Universities, 2019, 40(12): 2436. |
| [15] | WAN Hai, ZHANG Xiaoyu, ZHANG Ruojie, ZHANG Xiaotong, SONG Lijuan. Synthesis of High Performance ZSM-5-L Composite Zeolite and Its Catalytic Properities for n-Pentane Aromatization† [J]. Chem. J. Chinese Universities, 2014, 35(10): 2220. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||