

Chem. J. Chinese Universities ›› 2020, Vol. 41 ›› Issue (2): 284.doi: 10.7503/cjcu20190496
• Physical Chemistry • Previous Articles Next Articles
LI Bowen,WANG Ruoheng,LI Li,XIAO Yang( )
)
Received:2019-09-19
Online:2020-02-10
Published:2019-10-29
Contact:
Yang XIAO
E-mail:xiaoyang@scuec.edu.cn
Supported by:CLC Number:
TrendMD:
LI Bowen,WANG Ruoheng,LI Li,XIAO Yang. Adsorption of Toluene by Alkali Activated Porous Carbons and Activation/Adsorption Mechanism †[J]. Chem. J. Chinese Universities, 2020, 41(2): 284.
| Sample | Microporosity(%) | ||||
|---|---|---|---|---|---|
| SPCN-0 | 316.17 | 0.273 | 0.127 | 46.52 | 3.453 |
| SPCN-1 | 710.44 | 0.564 | 0.256 | 45.39 | 3.175 |
| SPCN-2 | 664.70 | 0.536 | 0.225 | 41.92 | 3.635 |
| SPCN-3 | 625.23 | 0.438 | 0.193 | 44.09 | 3.854 |
| SPCN-4 | 518.29 | 0.442 | 0.208 | 47.06 | 3.412 |
| Sample | Microporosity(%) | ||||
|---|---|---|---|---|---|
| SPCN-0 | 316.17 | 0.273 | 0.127 | 46.52 | 3.453 |
| SPCN-1 | 710.44 | 0.564 | 0.256 | 45.39 | 3.175 |
| SPCN-2 | 664.70 | 0.536 | 0.225 | 41.92 | 3.635 |
| SPCN-3 | 625.23 | 0.438 | 0.193 | 44.09 | 3.854 |
| SPCN-4 | 518.29 | 0.442 | 0.208 | 47.06 | 3.412 |
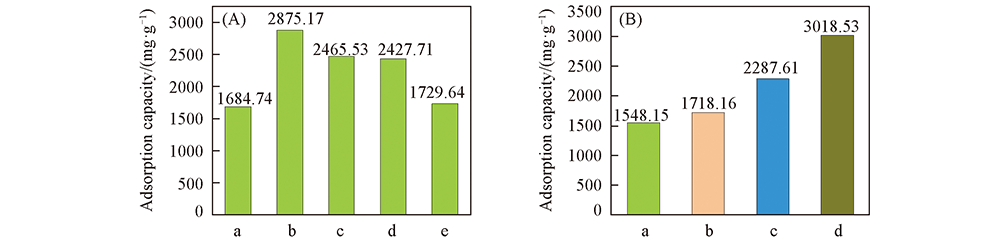
Fig.5 Toluene adsorption capacity of samples and adsorption capacity of SPCN-1 compared to silica gel, 10X molecular sieve and activated carbon particles (A) a. SPCN-0; b. SPCN-1; c. SPCN-2; d. SPCN-3; e. SPCN-4. (B) a. Molecular sieve; b. silica gel; c. activated carbon; d. SPCN-1.
| [1] | Moret S., Scolaro M., Barp L., Purcaro G., Sander M., Conte L. S., Food Chem., 2014,157, 470— 475 |
| [2] | Urlaub J., Norwig J., Schollmayer C., Holzgrabe U ., J. Pharm. Biomed. Anal., 2019,169, 41— 48 |
| [3] | Nash J. F., Gettings S. D., Diembeck W., Chudowski M., Kraus A. L., Food Chem. Toxicol., 1996,34(2), 213— 225 |
| [4] | Wagner M., Oellig C ., J. Chromatogr. A, 2019,1588, 48— 57 |
| [5] | Rawlings A. V., Lombard K. J., Int. J. Cosmet. Sci., 2012,34(6), 511— 518 |
| [6] | Kimber I., Carrillo J. C ., Toxicology, 2016, 344—346, 19— 25 |
| [7] | Biles R. W., Mckee R. H., Lewis S. C., Scala R. A., DePass L. R., Toxicology, 1988,53, 301— 314 |
| [8] |
Mackerer C. R., Griffis L. C., Grabowski J. S. Jr., Reitman F. A., Appl. Occup. Environ. Hyg., 2003,18(11), 890— 901
doi: 10.1080/10473220390237467 URL |
| [9] |
Garcia-Cicourel A. R., Janssen H. G., J. Chromatogr. A, 2019,1590, 113— 120
doi: 10.1016/j.chroma.2019.01.015 URL |
| [10] |
Zhou X., Qiu S. L., Liu L. X., Xing W. Y., He L. X., Hou Y. B., Fang M. Q., Gui Z., Song L., Hu Y., Composites Part B, 2019,167, 599— 607
doi: 10.1016/j.compositesb.2019.03.019 URL |
| [11] |
Petry T., Bury D., Fautz R., Hauser M., Huber B., Markowetz A., Mishra S., Rettinger K., Schuh W., Teichert T., Toxicol. Lett., 2017,280, 70— 78
doi: 10.1016/j.toxlet.2017.07.899 URL |
| [12] |
Tennant D. R., Food Chem. Toxicol., 2004,42(3), 481— 492
doi: 10.1016/j.fct.2003.10.011 URL |
| [13] | Nussbaum M. L., Knaggs E. A ., Process for Sulfonation, US 4148821, 1979-04-10 |
| [14] | Joseph M., Dibijak J., Griffith I ., Production of Technical White Mineral Oil, US 3629096, 1971-12-21 |
| [15] | Rausch M. K., , Making a White Oil by Two Stage of Catalytic Hydrogenation, US 3459656A 1969-08-05 |
| [16] |
Nugent P., Belmabkhout Y., Burd S. D., Cairns A. J., Luebke R., Forrest K., Pham T., Ma S., Space B., Wojtas L., Eddaoudi M., Zaworotko M. J., Nature, 2013,495(7439), 80— 84
doi: 10.1038/nature11893 URL |
| [17] |
Woellner M., Hausdorf S., Klein N., Mueller P., Smith M. W., Kaskel S., Adv. Mater., 2018,30(37), 1704679
doi: 10.1002/adma.v30.37 URL |
| [18] |
Yagub M. T., Sen T. K., Afroze S., Ang H. M., Adv. Colloid Interface Sci., 2014,209, 172— 184
doi: 10.1016/j.cis.2014.04.002 URL |
| [19] |
Ma S., Sun D., Yuan D., Wang X. S., Zhou H. C., J. Am. Chem. Soc., 2009,131(18), 6445— 6451
doi: 10.1021/ja808896f URL |
| [20] |
Kresge C. T., Roth W. J., Chem. Soc. Rev., 2013,42(9), 3663— 3670
doi: 10.1039/c3cs60016e URL |
| [21] |
Wurzbacher J. A., Gebald C., Steinfeld A., Energy Environ. Sci., 2011,4(9), 3584— 3592
doi: 10.1039/c1ee01681d URL |
| [22] |
Chen J., Qu R., Zhang Y., Sun C., Wang C., Ji C., Yin P., Chen H., Niu Y., Chem. Eng. J., 2012,209, 235— 244
doi: 10.1016/j.cej.2012.08.030 URL |
| [23] |
Zou H., Wu S., Shen J., Chem. Rev., 2008,108(9), 3893— 3957
doi: 10.1021/cr068035q URL |
| [24] |
Tanhaei B., Ayati A., Lahtinen M., Sillanpää M., Chem. Eng. J., 2015,259, 1— 10
doi: 10.1016/j.cej.2014.07.109 URL |
| [25] |
Cai W., Yu J., Jaroniec M ., J. Mater. Chem., 2010,20(22), 4587— 4594
doi: 10.1039/b924366f URL |
| [26] |
Hamon L., Llewellyn P. L., Devic T., Ghoufi A., Clet G., Guillerm V., Pirngruber G. D., Maurin G., Serre C., Driver G., van Beek W., Jolimaitre E., Vimont A., Daturi M., Ferey G., J. Am. Chem. Soc., 2009,131(47), 17490— 17499
doi: 10.1021/ja907556q URL |
| [27] |
An J., Rosi N. L., J. Am. Chem. Soc., 2010,132(16), 5578— 5579
doi: 10.1021/ja1012992 URL |
| [28] |
Xue D. X., Belmabkhout Y., Shekhah O., Jiang H., Adil K., Cairns A. J., Eddaoudi M., J. Am. Chem. Soc., 2015,137(15), 5034— 5040
doi: 10.1021/ja5131403 URL |
| [29] | Suo L. L., Juan S., Tao L., Zhang L., Zhang X., Chem. J. Chinese Universities, 2016,37(11), 2043— 2049 |
| ( 索路路, 李生娟, 李应涛, 张莉, 张熙 . 高等学校化学学报, 2016,37(11), 2043— 2049) | |
| [30] |
He X., Male K. B., Nesterenko P. N., Brabazon D., Paull B., Luong J. H., ACS Appl. Mater. Interfaces, 2013,5(17), 8796— 8804
doi: 10.1021/am403222u URL |
| [31] |
Wickramaratne N. P., Xu J., Wang M., Zhu L., Dai L., Jaroniec M., Chem. Mater., 2014,26(9), 2820— 2828
doi: 10.1021/cm5001895 URL |
| [32] |
Zhu X., Liu Y., Zhou C., Luo G., Zhang S., Chen J ., Carbon, 2014,77, 627— 636
doi: 10.1016/j.carbon.2014.05.067 URL |
| [33] |
Siyasukh A., Chimupala Y., Tonanon N ., Carbon, 2018,134, 207— 221
doi: 10.1016/j.carbon.2018.03.093 URL |
| [34] |
Lee J., Kim J., Hyeon T., Adv. Mater., 2006,18(16), 2073— 2094
doi: 10.1002/(ISSN)1521-4095 URL |
| [35] |
Liang C., Li Z., Dai S., Angew. Chem. Int. Ed., 2008,47(20), 3696— 3717
doi: 10.1002/(ISSN)1521-3773 URL |
| [36] |
Stein A., Wang Z., Fierke M. A., Adv. Mater., 2009,21(3), 265— 293
doi: 10.1002/adma.v21:3 URL |
| [37] |
Sevilla M., Valle-Vigón P., Fuertes A. B., Adv. Funct. Mater., 2011,21(14), 2781— 2787
doi: 10.1002/adfm.201100291 URL |
| [38] |
Dutta S., Bhaumik A., Wu K. C. W., Energy Environ. Sci., 2014,7(11), 3574— 3592
doi: 10.1039/C4EE01075B URL |
| [39] |
Luna F. M. T., Pontes-Filho A. A., Trindade E. D., Silva I. J., Azevedo D. C. S., Cavalcante C. L., Ind. Eng. Chem. Res., 2008,47(9), 3207— 3212
doi: 10.1021/ie071476v URL |
| [40] |
Milczarek G., Inganas O ., Science, 2012,335(6075), 1468— 1471
doi: 10.1126/science.1215159 URL |
| [41] |
Xie A., Dai J., Chen X., Ma P., He J., Li C., Zhou Z., Yan Y., Chem. Eng. J., 2016,304, 609— 620
doi: 10.1016/j.cej.2016.06.138 URL |
| [42] |
Saito T., Brown R. H., Hunt M. A., Pickel D. L., Pickel J. M., Messman J. M., Baker F. S., Keller M., Naskar A. K., Green Chem., 2012,14(12), 3295— 3303
doi: 10.1039/c2gc35933b URL |
| [43] |
Lillo-Ródenas M. A., Lozano-Castelló D., Cazorla-Amorós D., Linares-Solano A., Carbon, 2001,39, 751— 759
doi: 10.1016/S0008-6223(00)00186-X URL |
| [44] |
Lillo-Ródenas M. A., Juan-Juan J., Cazorla-Amorós D., Linares-Solano A., Carbon, 2004,42(7), 1371— 1375
doi: 10.1016/j.carbon.2004.01.008 URL |
| [45] |
Lillo-Ródenas M. A., Cazorla-Amorós D., Linares-Solano A., Carbon, 2003,41, 267— 275
doi: 10.1016/S0008-6223(02)00279-8 URL |
| [46] |
Raymundo-Piñero E., Azaïs P., Cacciaguerra T., Cazorla-Amorós D., Linares-Solano A., Béguin F ., Carbon, 2005,43(4), 786— 795
doi: 10.1016/j.carbon.2004.11.005 URL |
| [47] |
Foo K. Y., Hameed B. H., Chem. Eng. J., 2012,187, 53— 62
doi: 10.1016/j.cej.2012.01.079 URL |
| [48] | Chen H., Wang H., Xue Z., Yang L., Xiao Y., Zheng M., Lei B., Liu Y., Sun L., Int. J. Hydrogen Energy, 2012,37(24), 18888— 18894 |
| [49] | Byamba-Ochir N., Shim W. G., Balathanigaimani M. S., Moon H., Appl. Surf. Sci., 2016,379, 331— 337 |
| [50] | Yamashita Y., Ouchi K ., Carbon, 1982,20(1), 41— 45 |
| [51] | Yamashita Y., Ouchi K ., Carbon, 1982,20(1), 47— 53 |
| [52] | Su F., Poh C. K., Chen J. S., Xu G., Wang D., Li Q., Lin J., Lou X. W., Energy Environ. Sci., 2011,4(3), 717— 724 |
| [53] | Qie L., Chen W. M., Wang Z. H., Shao Q. G., Li X., Yuan L. X., Hu X. L., Zhang W. X., Huang Y. H., Adv. Mater., 2012,24(15), 2047— 2050 |
| [54] | Chen Z., Ma L., Li S., Geng J., Song Q., Liu J., Wang C., Wang H., Li J., Qin Z., Li S., Appl. Surf. Sci., 2011,257(20), 8686— 8691 |
| [55] | Milczarek G ., Langmuir, 2009,25(17), 10345— 10353 |
| [56] |
Li X., Chen S., Fan X., Quan X., Tan F., Zhang Y., Gao J ., J. Colloid Interface Sci., 2015,447, 120— 127
doi: 10.1016/j.jcis.2015.01.042 URL |
| [57] |
Si Y., Ren T., Li Y., Ding B., Yu J ., Carbon, 2012,50(14), 5176— 5185
doi: 10.1016/j.carbon.2012.06.059 URL |
| [58] | Thomas W. J., Crittenden B., Adsorption Technology and Design, Butterworth-Heinemann Oxford, 1998, 66—86, 146— 162 |
| [59] |
Yuan M., Tong S., Zhao S., Jia C. Q., J. Hazard. Mater., 2010,181(1—3), 1115— 1120
doi: 10.1016/j.jhazmat.2010.05.130 URL |
| [60] |
Cardoso N. F., Pinto R. B., Lima E. C., Calvete T., Amavisca C. V., Royer B., Cunha M. L., Fernandes T. H. M., Pinto I. S., Desalination, 2011,269(1—3), 92— 103
doi: 10.1016/j.desal.2010.10.047 URL |
| [61] |
Chang B., Guan D., Tian Y., Yang Z., Dong X ., J. Hazard. Mater., 2013,262(15), 256— 264
doi: 10.1016/j.jhazmat.2013.08.054 URL |
| [62] | Polanyi M., Verh. Deutsch. Phys . Ges., 1914,16, 1012 |
| [63] |
Manes M., Hofer L. J. E., J. Phys. Chem., 1969,73(3), 584— 590
doi: 10.1021/j100723a018 URL |
| [64] |
Ji L., Wan Y., Zheng S., Zhu D., Environ. Sci. Technol., 2011,45(13), 5580— 5586
doi: 10.1021/es200483b URL |
| [65] |
Ji L., Chen W., Duan L., Zhu D., Environ. Sci. Technol., 2009,43(7), 2322— 2327
doi: 10.1021/es803268b URL |
| [66] |
Bernal V., Giraldo L., Moreno-Pirajan J. C., Balsamo M., Erto A., Molecules, 2019,24(3), 413— 433
doi: 10.3390/molecules24030413 URL |
| [67] |
McGaughey G. B., Gagné M., Rappé A. K., J. Biol. Chem., 1998,273(25), 15458— 15463
doi: 10.1074/jbc.273.25.15458 URL |
| [68] |
Sinnokrot M. O., Valeev E. F., Sherrill C. D., J. Am. Chem. Soc., 2002,124(36), 10887— 10893
doi: 10.1021/ja025896h URL |
| [69] |
Moreno-Castilla C ., Carbon, 2004,42(1), 83— 94
doi: 10.1016/j.carbon.2003.09.022 URL |
| [70] |
Mattson J. S., Mark H. B., J. R., Malbin M. D., Weber W. J., Crittenden J. C., J. Colloid Interface Sci., 1969,31(1), 116— 130
doi: 10.1016/0021-9797(69)90089-7 URL |
| [1] | HAO Honglei, MENG Fanyu, LI Ruoyu, LI Yingqiu, JIA Mingjun, ZHANG Wenxiang, YUAN Xiaoling. Biomass Derived Nitrogen Doped Porous Carbon Materials as Adsorbents for Removal of Methylene Blue in Water [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220055. |
| [2] | LUO Bian, ZHOU Fen, PAN Mu. Study on Preparation and Accessibility of Hierarchical Porous Carbon Supported Platinum Catalyst [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210853. |
| [3] | FANG Jiyong, JIANG Zhenhua, YUE Xigui. Preparation and Properties of Polyaryletherketones-based Electromagnetic Wave Absorption Composite Material [J]. Chem. J. Chinese Universities, 2021, 42(6): 1994. |
| [4] | WANG Changyao, WANG Shuai, DUAN Linlin, ZHU Xiaohang, ZHANG Xingmiao, LI Wei. In situ Confinement Growth Strategy for Ordered Mesoporous Carbon Support Ultrasmall MoO3 Nanoparticles [J]. Chem. J. Chinese Universities, 2021, 42(5): 1589. |
| [5] | LI Min, ZHAO Chun, FENG Qinzhong, FENG Jian, MENG Xiaojing. Experimental and DFT Studies on the Adsorption of Cd(II) Ions from Aqueous Solutions by Nanofiber Modified Thiourea Groups [J]. Chem. J. Chinese Universities, 2021, 42(12): 3680. |
| [6] | YU Jianxing, YU Moxin, KUAI Le, ZHU Bowen. Preparation of High Oxygen Porous Carbon from Walnut Peel and Its Adsorption Properties for Ni2+ [J]. Chem. J. Chinese Universities, 2020, 41(11): 2464. |
| [7] | LI Ze, WANG Jianjiang, GAO Haitao, ZHAO Fang. Fabrication and Microwave Absorption Mechanism of PCIP/CoFe2O4/PANI Composites [J]. Chem. J. Chinese Universities, 2019, 40(8): 1784. |
| [8] | Ying MA,Tian WANG,Heng ZHANG. Molecular Dynamics Simulation of Adsorption of Methylene Blue by Graphene Oxide † [J]. Chem. J. Chinese Universities, 2019, 40(12): 2534. |
| [9] | WEI Junyi, GAO Zhihua, HUANG Wei, AI Peipei, YAN Feifei, YOU Xiangxuan. Effect of Structural Ordering on the Performance of Mesoporous Carbon Supported CuCoCe Catalyst in the Synthesis of Higher Alcohols from Syngas† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1741. |
| [10] | WANG Xiuli, HE Xingquan. Electrocatalytic Performance of Fe9S10 Nanoparticles Loaded Nitrogen and Sulphur Codoped Porous Carbon for Oxygen Reduction Reaction† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1524. |
| [11] | CHENG Yanyan, SUI Guanghui, CHEN Zhimin, LIU Huan, DUAN Yajun, WANG Xiaofeng, YANG Xiaomin, WANG Zichen. Highly Efficient Utilization of Rice Husk Char to Prepare Carbon Adsorbent and Calcium Silicate† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1132. |
| [12] | LUAN Jingde, LIU Yawei, ZHANG Chengyu, KE Xin. Synchronization Capture of Zn2+ and p-Nitrophenyl Phenol on Montmorillonite Composites† [J]. Chem. J. Chinese Universities, 2018, 39(2): 270. |
| [13] | WANG Xiaobing, SHI Xiuyi, ZHOU Xingjun, QIU Xiuzhen, LU Wenguan. Adsorption Behavior of Metal-organic Framework NH2-MIL-53(Al) for Diclofenac Sodium in Aqueous Solution† [J]. Chem. J. Chinese Universities, 2018, 39(2): 206. |
| [14] | ZHANG Baohai, LUO Min, YANG Shun, FU Rongrong, MA Jinfu. Preparation and Electrochemical Properties of Hierarchically Porous Carbon Microspheres Derived from Metal Phenolic Precursor† [J]. Chem. J. Chinese Universities, 2018, 39(2): 310. |
| [15] | XU Xiaomu, ZHANG Xingshuai, CHEN Zhining, WANG Jing, GUO Yuzhong, HUANG Ruian, WANG Jianhua. Preparation of a Disordered Mesoporous Silicon Nanocomposite and Its Slow Activation Behavior† [J]. Chem. J. Chinese Universities, 2017, 38(5): 713. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||