

Chem. J. Chinese Universities ›› 2020, Vol. 41 ›› Issue (2): 277.doi: 10.7503/cjcu20190380
• Organic Chemistry • Previous Articles Next Articles
JIANG Jing1,2,CHEN Xiaoli3,HUANG Yali3,ZHANG Qilong1,3,*( ),XU Hong3,*(
),XU Hong3,*( ),YANG Xiaosheng1
),YANG Xiaosheng1
Received:2019-07-06
Online:2020-02-10
Published:2019-12-04
Contact:
Qilong ZHANG,Hong XU
E-mail:gzuqlzhang@126.com;1738943269@qq.com
Supported by:TrendMD:
JIANG Jing,CHEN Xiaoli,HUANG Yali,ZHANG Qilong,XU Hong,YANG Xiaosheng. Interaction Mode Between Q[8] and Feb †[J]. Chem. J. Chinese Universities, 2020, 41(2): 277.
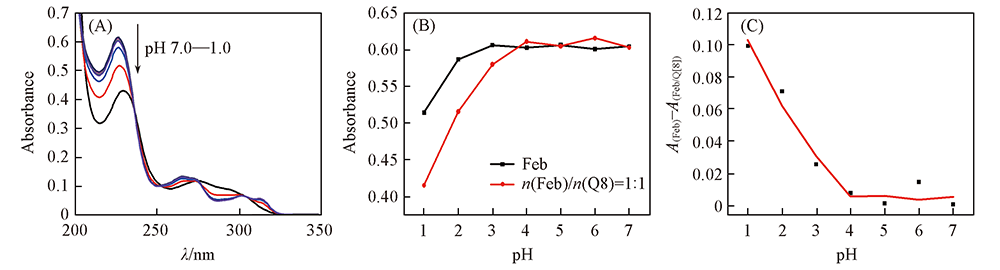
Fig.2 UV spectra of Feb with Q[8] at different pH values(A), trend chart of simple Feb solution and after addition the Q[8](B) and ΔA value(C) of AFeb and AFeb/Q[8] λ=228 nm; c(Feb)=20 μmol/L.
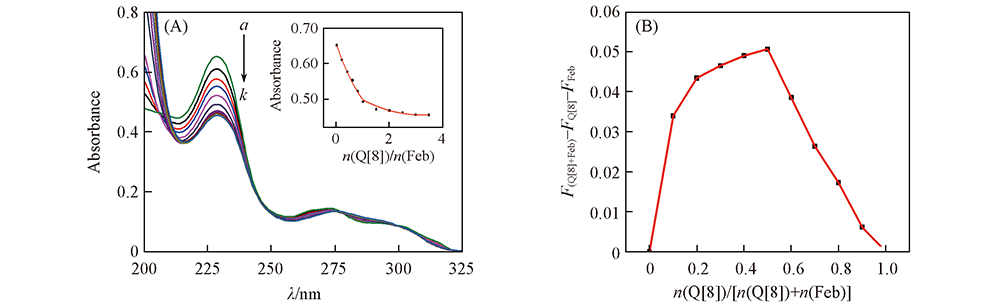
Fig.3 UV spectra(A) and Job’s plot(B) of Feb with Q[8] (A) c(Feb)=20 μmol/L; n(Q[8])/n(Feb), a—k: 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5. Inset of (A): absorption intensity plot at 228 nm of Feb upon addition of increasing concentrations of Q[8].
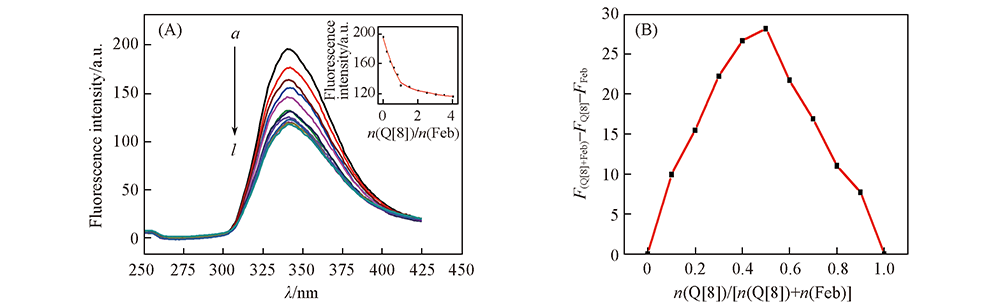
Fig.4 Fluorescence emission spectra(A), and Job’s plot(B) of Feb with Q[8] (A) c(Feb)=20 μmol/L; n(Q[8])/n(Feb), a—l: 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0. Inset of (A): the fluorescence intensity plot at 340 nm of Feb upon addition of increasing concentrations of Q[8].
| [1] | Chinese Pharmacopoeia Commission, Chinese Pharmacopoeia 2015, Section One, China Medical Science and Technology Press, Beijing, 2015,313 |
| ( 国家药典委员会. 中国药典2015年版一部, 北京: 中国医药科技出版社, 2015,313) | |
| [2] | Li Y., Liu M. C., Jin L. H., Hu D. Y., Yang S., Guangzhou Chemical Industry, 2011,39(9), 7— 9 |
| ( 李燕, 刘明川, 金林红, 胡德禹, 杨松 . 广州化工, 2011,39(9), 7— 9) | |
| [3] | Guo Z. T., Liu X. L., Liang J. P., Shang R. F., Liu Y., Qiang Z., Journal of Traditional Chinese Veterinary Medicine, 2013,32(2), 17— 19 |
| ( 郭志廷, 刘晓璐, 梁剑平, 尚若锋, 刘宇, 强哲 . 中兽医医药杂志, 2013,32(2), 17— 19) | |
| [4] | Zhang J. Y., Liu X. Q., Yang L. X., Feng W. H., China Journal of Chinese Materia Medica, 2017,42(16), 3178— 3184 |
| ( 张继远, 刘晓谦, 杨立新, 冯伟红 . 中国中药杂志, 2017,42(16), 3178— 3184) | |
| [5] | Zhu S., Chandrashekar G., Meng L., Robinson K., Chatterji D., Bioorg. Med. Chem., 2012,20(2), 927— 932 |
| [6] |
Kaur K., Jain M., Kaur T., Jain R., Bioorg. Med. Chem., 2009,17(9), 3229— 3256
doi: 10.1016/j.bmc.2009.02.050 URL |
| [7] | Gao Z. R., Luo Y., Su K. X., Pan J. C., Xie X. Y., Gao Y. J., Chinese Journal of Practical Stomatology, 2018,11(6), 343— 346 |
| ( 高峥嵘, 罗银, 苏楷欣, 潘军臣, 谢小燕, 高义军 . 中国实用口腔科杂志, 2018,11(6), 343— 346) | |
| [8] |
Jonge M. J. A. D., Dumez H., Verweij J., Yarkoni S., Snyder D., Lacombe D., Marréaud S., Yamaguchi T., Punt C. J. A., van Oosterom A., Eur. J. Cancer, 2006,42(12), 1768— 1764
doi: 10.1016/j.ejca.2005.12.027 URL |
| [9] | Guo Z. Y., Liang J. P., Wei X. B., Shang R. F., Guo W. Z., Wang X. H., Hao B. C., Chinese Journal of Veterinary Science, 2013,33(7), 1083— 1085, 1118 |
| ( 郭志廷, 梁剑平, 韦旭斌, 尚若锋, 郭文柱, 王学红, 郝宝成 . 中国兽医学报, 2013,33(7), 1083— 1085, 1118) | |
| [10] | Jiang W. D., Acta Physiologica Sinica, 1961,24(3), 180— 186 |
| ( 江文德 . 生理学报, 1961,24(3), 180— 186) | |
| [11] | Nanjing University Of Chinese Medicine, Chinese Medicine Dictionary Volume Ⅱ(Second Edition), Shanghai Scientific and Technical Publishers, Shanghai, 2006, 2942— 2944 |
| ( 南京中医药大学. 中药大辞典下册(第2版), 上海: 上海科学技术出版社, 2006, 2942— 2944) | |
| [12] |
Assaf K. I., Nau W. M., Chem. Soc. Rev., 2014,44(2), 394— 418
doi: 10.1039/C4CS00273C URL |
| [13] |
Mandadapu V., Day A. I., Ghanem A., Chirality, 2014,26(11), 712— 723
doi: 10.1002/chir.22363 URL |
| [14] |
Day A. I. D., Blanch R. J. B., Arnold A. P., Lorenzo S., Lewis G. R., Dance I., Angew. Chem. Int. Ed. Engl., 2010,41(2), 275— 277
doi: 10.1002/1521-3773(20020118)41:2<275::AID-ANIE275>3.0.CO;2-M URL |
| [15] | Huang Y., Tao Z., Xue S. F., Zhu Q. J., Chem. J. Chinese Universities, 2011,32(9), 2022— 2031 |
| ( 黄英, 陶朱, 薛赛凤, 祝黔江 . 高等学校化学学报, 2011,32(9), 2022— 2031) | |
| [16] |
Uzunova V. D., Cullinane C., Brix K., Nau W. M., Day A. I., Org. Biomol. Chem., 2010,8(9), 2037— 2042
doi: 10.1039/b925555a URL |
| [17] |
Chen H., Chan J. Y. W., Xue Y., Wyman I. W., Bardelang D., Macartney D. H., Lee S. M. Y., Wang R., RSC Advances, 2015,5(38), 30067— 30074
doi: 10.1039/C5RA04335B URL |
| [18] | Fu Y. Z., Shen X. C., Huang Y., Tao Z., Xue S. F., Zhu Q. J., Journal of Guizhou University(Natural Sciences), 2007,24(6), 650— 652 |
| ( 傅晓钟, 沈祥春, 黄英, 陶朱, 薛赛凤, 祝黔江 . 贵州大学学报(自然科学版), 2007,24(6), 650— 652) | |
| [19] | Du P., Zhang Y. P., Journal of Guiyang University of Chinese Medicine, 2016,38(6), 9— 11 |
| ( 杜鹏, 张永萍 . 贵阳中医学院学报, 2016,38(6), 9— 11) | |
| [20] | Jiang J., Huang Y. L., Zhang Q. L., Xu H., Sun X. H., Chem. J. Chinese Universities, 2019,40(1), 76— 82 |
| ( 蒋静, 黄亚励, 张奇龙, 徐红, 孙晓红 . 高等学校化学学报, 2019,40(1), 76— 82) | |
| [21] |
Held B., Tang H., Natarajan P., Da S. C., De O. S. V., Bohne C., Quina F. H., Photochem. Photobiol. Sci., 2016,15(6), 832— 832
doi: 10.1039/C6PP90018F URL |
| [22] |
Basílio N., Petrov V., Pina F . ChemPlusChem, 2016,80(12), 1779— 1785
doi: 10.1002/cplu.201500304 URL |
| [23] | Chinese Pharmacopoeia Commission, Chinese Pharmacopoeia, 2015, Section Two, China Medicai Science and Technology Press, Beijing, 2015,119 |
| ( 国家药典委员会. 中国药典 2015年版二部, 北京: 中国医药科技出版社, 2015,119) |
| [1] | FANG Chao,ZHU Hanyu,LIU Ye,ZHAO Waiou,LI Yapeng,WANG Jingyuan. Synthesis and Characterization of Nanoparticles with Hydrogen Peroxide Sensitivity, Targeting and Fluorscence for Atherosclerosis [J]. Chem. J. Chinese Universities, 2018, 39(9): 2071. |
| [2] | YE Hui, LIU Yabo, JIA Yuxi. Numerical Simulation of Swelling and Drug Release Processes for Weak Polyelectrolyte Hydrogels† [J]. Chem. J. Chinese Universities, 2018, 39(4): 817. |
| [3] | JIAN Yuhang, YAN Shifeng, LI Xing, HUANG Yanan, YIN Jingbo. Synthesis and Characterization of Injectable Hydrogels Based on Star β-CD-g-poly(L-glutamic acid) [J]. Chem. J. Chinese Universities, 2017, 38(8): 1489. |
| [4] | ZHANG Haipeng, HAN Bing, JIA Zhizhen, DING Rongbo, XU Bin, XU Weiqing, FAN Zhimin. Fabrication of Drug Loaded Fluorescent Nanoparticles and Its Biological Application in MCF-7 Breast Cancer Cell [J]. Chem. J. Chinese Universities, 2017, 38(5): 860. |
| [5] | WANG Xiaodan, XU Dandan, LÜ Weizhong, LIU Jingyuan, LIU Qi, JING Xiaoyan, WANG Jun. Preparation and Drug Release of Anti-cancer Fe3O4@ZIF-8@PA System Loaded with Drug† [J]. Chem. J. Chinese Universities, 2017, 38(11): 1927. |
| [6] | LI Zhengzheng, XU Ziyang, GAO Liuyi, ZENG Wei, ZHAO Linlin. Preparation and Characterization of Thermo-sensitive N-Acetyl Glycol Chitosan Hydrogel for Sustained Drug Release† [J]. Chem. J. Chinese Universities, 2016, 37(12): 2299. |
| [7] | ZHU Shoujin, LIU Faqian, WANG Jingzhao, SU Feng, LI Suming. Systhesis and Characterization of Novel Carboxymethyl Chitosan Hydrogel† [J]. Chem. J. Chinese Universities, 2014, 35(4): 863. |
| [8] | WEI Yu, JI Ying, JI Jian. REDV/Peptide Conjugated Rapamycin-loaded Polymer Matrix for Endothelial Cells Selectivity [J]. Chem. J. Chinese Universities, 2012, 33(01): 193. |
| [9] | NIE Xin, QU Feng-Yu, LI Xiao-Feng, LIN Hui-Ming. Synthesis of Spherical Mesoporous SBA-15 with Abundant Carboxyl and Drug Release Profile [J]. Chem. J. Chinese Universities, 2011, 32(7): 1478. |
| [10] | CHEN Yang-Juan, ZHONG Shi-An*, SHI Qiong. Synthesis of Degradable Poly(polyamidoamine-methacrylamide-g-anhydride) Photocrosslinked Gels and Their Controlled Drug Release Behavior [J]. Chem. J. Chinese Universities, 2011, 32(5): 1194. |
| [11] | HAN Ya-Dong, XIA Jia-Liang, HE Pan, TIAN Hua-Yu, CHEN Xue-Si*, JING Xia-Bin. Poly(α,L-glutamic acid) Microspheres for Oral Insulin Delivery [J]. Chem. J. Chinese Universities, 2009, 30(12): 2521. |
| [12] | MAO Jing, GAN Zhi-Hua*. Synthesis and Controlled Drug Release of Amphiphilic Graft Copolymers PEO-b-PGL-g-PCL [J]. Chem. J. Chinese Universities, 2009, 30(11): 2291. |
| [13] | WANG Xin1, WU Zhong-Ming2, ZHANG Xin-Ge1, ZHENG Chao1, WANG Zhen1, LI Chao-Xing1*. Properties and in vitro Evaluation of Chitosan-NAC Nanoparticle for Drug Release [J]. Chem. J. Chinese Universities, 2008, 29(4): 851. |
| [14] | YU Yang1, YIN Jing-Bo1*, LUO Kun1, XIE Yong-Tao1, YAN Shi-Feng1, MA Jia2, CHEN Xue-Si2*. Preparation of Temperature- and pH-Sensitive PVME/CMCS Hydrogels via Electron Beam Irradiation Cross-linking and Their Properties [J]. Chem. J. Chinese Universities, 2008, 29(2): 409. |
| [15] | TANG Yu, WANG Zhen, LI Chao-Xing*. Preparation of a Novel Amphiphilic Copolymer Microspheres with Phenylborate Moieties and Its Glucose-sensitive Properties [J]. Chem. J. Chinese Universities, 2007, 28(8): 1581. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||