

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (1): 41.doi: 10.7503/cjcu20180641
• Analytical Chemistry • Previous Articles Next Articles
BAI Lei, HUO Shuhui, CHEN Jing, LU Xiaoquan*( )
)
Received:2018-09-14
Online:2019-01-10
Published:2018-11-28
Contact:
LU Xiaoquan
E-mail:luxq@nwnu.edu.cn
Supported by:CLC Number:
TrendMD:
BAI Lei,HUO Shuhui,CHEN Jing,LU Xiaoquan. Squaramide Fluorescence Probe for Chiral Recognition of α-Amino Acids†[J]. Chem. J. Chinese Universities, 2019, 40(1): 41.
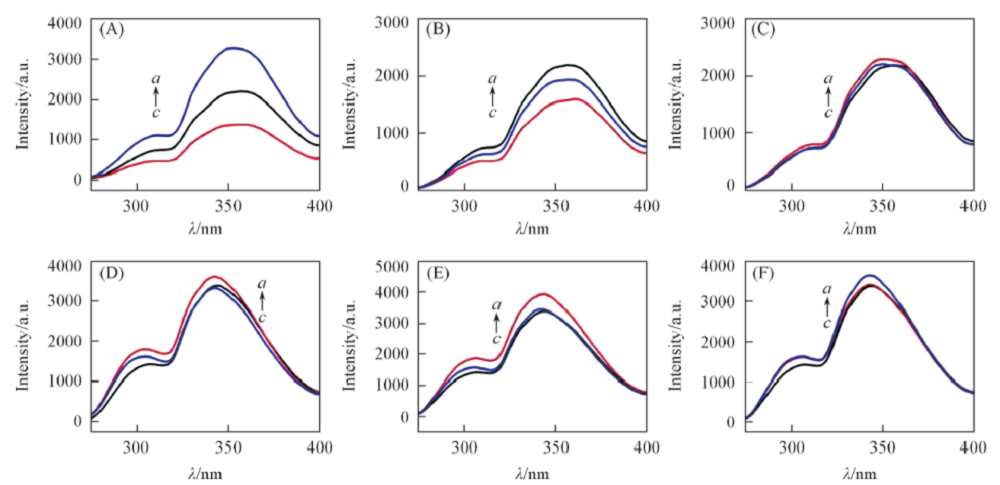
Fig.1 Fluorescence spectra of probes 5 and 6 with and without α-amino acid(A) a. L-Phe+probe 5; b. probe 5; c. D-Phe+probe 5; λex=230 nm, slit=5 nm/5 nm; (B) a. probe 5; b. L-Val+probe 5; c. D-Val+probe 5; λex=230 nm, slit=5 nm/5 nm; (C) a. D-Pro+probe 5; b. L-Pro+probe 5; c. probe 5; λex=230 nm, slit=5 nm/5 nm; (D) a. D-Phe+probe 6; b. probe 6; c. L-Phe+probe 6; λex=225 nm, slit=5 nm/5 nm; (E) a. D-Val+probe 6; b. L-Val+probe 6; c. probe 6; λex=225 nm, slit=5 nm/5 nm; (F) a. L-Pro+probe 6; b. D-Pro+Probe 6; c. probe 6; λex=225 nm, slit=5 nm/5 nm.
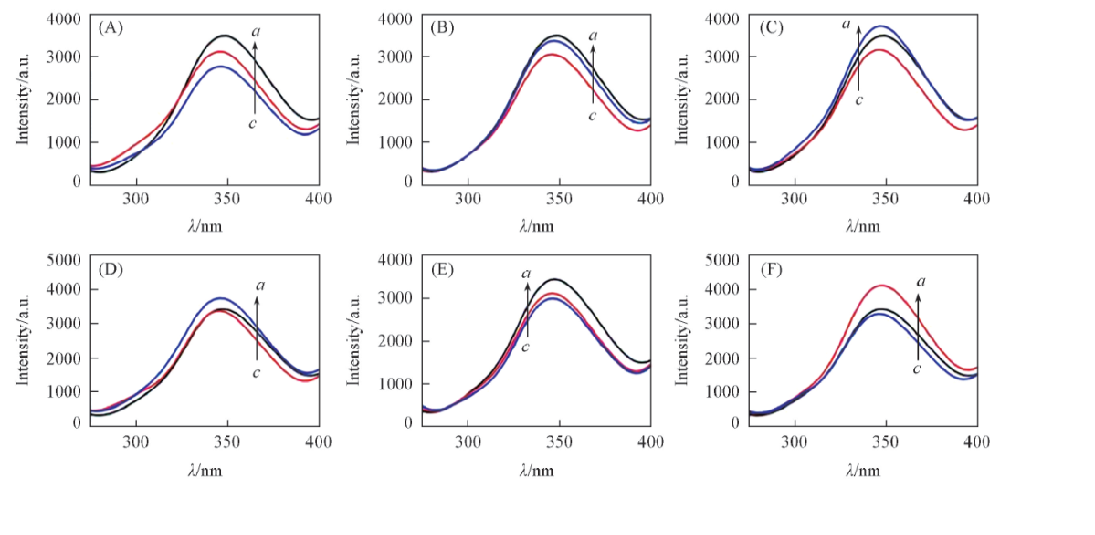
Fig.2 Fluorescence spectra of probes 7 and 8 with and without α-amino acid(A) a. Probe 7; b. D-Phe+probe 7; c. L-Phe+probe 7; λex=230 nm, slit=20 nm/20 nm; (B) a. Probe 7; b. L-Val+probe 7; c. D-Val+probe 7; λex=230 nm, slit=20 nm/20 nm; (C) a. L-Pro+probe 7; b. probe 7; c. D-Pro+probe 7. λex=230 nm, slit=20 nm/20 nm; (D) a. L-Phe+probe 8; b. probe 8; c. D-Phe+probe 8; λex=230 nm, slit=20 nm/20 nm; (E) a. Probe 8; b. D-Val+probe 8; c. L-Val+probe 8; λex=230 nm, slit=20 nm/20 nm; (F) a. D-Pro+probe 8; b. probe 8; c. L-Pro+probe 8; λex=230 nm, slit=20 nm/20 nm.
| Probe | IL/ID or ID/IL | ||
|---|---|---|---|
| Phe | Val | Pro | |
| 5 | 2.4 | 1.2 | 1.0 |
| 6 | 1.1 | 1.1 | 1.0 |
| 7 | 1.1 | 1.1 | 1.1 |
| 8 | 1.1 | 1.0 | 1.3 |
Table 1 Fluorescence intensity ratios of probes 5—8 with amino acids
| Probe | IL/ID or ID/IL | ||
|---|---|---|---|
| Phe | Val | Pro | |
| 5 | 2.4 | 1.2 | 1.0 |
| 6 | 1.1 | 1.1 | 1.0 |
| 7 | 1.1 | 1.1 | 1.1 |
| 8 | 1.1 | 1.0 | 1.3 |
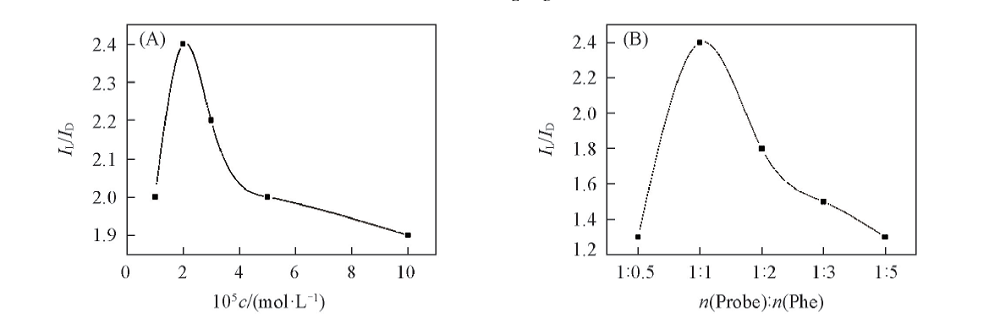
Fig.3 IL/ID plots of probe 5 at various concentrations of L-Phe or D-Phe at λem=358 nm(A) and IL/ID plots of probe 5 at various molar ratios of probe 5 to L-Phe or D-Phe at λem=358 nm(B)λex=230 nm, slit=5 nm/5 nm.
| [1] | Schurig V., Nowotny H. P., Angew. Chem. Int. Ed., 1990, 29, 939—957 |
| [2] | Zhang Z. C., Zhang K., Jiang Z. J., Zhou Q. L., Wang Q. S., Gao R. Y., Chem. J. Chinese Universities,2001, 22(1), 42—44 |
| (张智超, 张锴, 江正瑾, 周其林, 王琴孙, 高如瑜. 高等学校化学学报, 2001, 22(1), 42—44) | |
| [3] | Chen H., Lü X. Y., Huang J. M., Gao R. Y., Wang Q. S., Chem. J. Chinese Universities,2000, 21(4), 562—565 |
| (陈慧, 吕宪禹, 黄君珉, 高如瑜, 王琴孙. 高等学校化学学报, 2000, 21(4), 562—565) | |
| [4] | Huang J. M., Chen H., Gao R. Y., Wang Q. S., Chem. J. Chinese Universities,2001, 22(11), 1838—1842 |
| (黄君珉, 陈慧, 高如瑜, 王琴孙. 高等学校化学学报, 2001, 22(11), 1838—1842) | |
| [5] | Busschaert N., Caltagirone C., van Rossom W., Gale P. A., Chem. Rev.,2015, 115, 8038—8155 |
| [6] | Saleem M., Lee K.H., RSC Adv.,2015, 5, 72150—72287 |
| [7] | Dong W. K., Akogun S. F., Zhang Y., Sun Y. X., Dong X. Y., Sens. Actuators B,2017, 238, 723—734 |
| [8] | Zhou J. J., Song X. Q., Liu Y. A., Wang X. L., RSC Adv.,2017, 7, 25549—25559 |
| [9] | Yu B., Li C. Y., Sun Y. X., Jia H. R., Guo J. Q., Li J., Spectrochim. Acta A,2017, 184, 249—254 |
| [10] | Dong W. K., Li X. L., Wang L., Zhang Y., Ding Y. J., Sens. Actuators B,2016, 229, 370—378 |
| [11] | Hu J. H., Sun Y., Qi J., Li Q., Wei T. B., Spectrochim. Acta A,2017, 175, 125—133 |
| [12] | Xu Y., Mao S., Peng H., Wang F., Zhang H., Aderinto S. O., Wu H., J. Luminesc.,2017, 192, 56—63 |
| [13] | Pu L., Chem. Rev., 2004, 104, 1687—1716 |
| [14] | Pu L., Acc. Chem. Res., 2012, 45, 150—163 |
| [15] | Leung D., Kang S. O., Anslyn E. V., Chem. Soc. Rev.,2012, 41, 448—479 |
| [16] | Zhang X., Yin J., Yoon J., Chem. Rev.,2014, 114, 4918—4959 |
| [17] | Liu H. L., Peng Q., Wu Y. D., Chen D., Hou X. L., Sabat M., Pu L., Angew. Chem. Int. Ed.,2010, 49, 602—606 |
| [18] | Folmer-Andersen J. F., Lynch V. M., Anslyn E. V., J. Am. Chem. Soc.,2005, 127, 7986—7987 |
| [19] | Hu C., He Y., Chen Z., Huang X., Tetrahedron: Asymm.,2009, 20, 104—110 |
| [20] | Kim Y. K., Lee H. N., Singh N. J., Choi H. J., Xue J., Kim K. S., Yoon J., Hyun M. H., J. Org. Chem.,2008, 73, 301—304 |
| [21] | Upadhyay S. P., Pissurlenkar R. R. S., Coutinho E. C., Karnik A.V., J. Org.Chem.,2007, 72, 5709—5714 |
| [22] | Zhao J., Fyles T. M., James T. D., Angew. Chem. Int.Ed.,2004, 43, 3461—3464 |
| [23] | Gingter S., Bezdushna E., Ritter H., Macromolecules,2010, 43, 3128—3131 |
| [24] | Hao Y. Q., Wang L. X., Ma L. J., Wu Y. Q., Liu J. Q., Luo G. M., Yang G. D., Chem. J. Chinese Universities,2006, 27(5), 920—924 |
| (郝雅琼, 王立旭, 马立军, 吴玉清, 刘俊秋, 罗贵民, 杨光弟. 高等学校化学学报, 2006, 27(5), 920—924) | |
| [25] | Song F., Wei G., Wang L., Jiao J., Cheng Y., Zhu C., J. Org. Chem.,2012, 77, 4759—4764 |
| [26] | Folmer-Andersen J. F., Lynch V. M., Anslyn E. V., J. Am. Chem. Soc.,2005, 127, 7986—7987 |
| [27] | Chen X., Huang Z., Chen S., Li K., Yu X., Pu L., J. Am. Chem. Soc.,2010, 132, 7297—7299 |
| [28] | Albrecht Ł., Dickmeiss G., Acosta F. C., Rodríguez-Escrich C., Davis R. L., Jørgensen K. A., J. Am. Chem.Soc.,2012, 134, 2543—2546 |
| [29] | Malerich J. P., Hagihara K., Rawal V. H., J. Am. Chem. Soc.,2008, 130, 14416—14417 |
| [30] | Ni X., Wang Z., Li X., Cheng J. P., Org Lett.,2014, 16, 1786—1789 |
| [31] | Li Y., Yang G. H., He C. Q., Li X., Houk K. N., Cheng J. P., Org. Lett.,2017, 19, 4191—4194 |
| [32] | Yang G. H., Li Y., Li X., Asian J. Org. Chem.,2018, 7, 770—775 |
| [33] | Corradini R., Paganuzzi C., Marchelli R., Pagliari S., Sforza S., Dossena A., Galaverna G., Duchateau A., J. Mater. Chem.,2005, 15, 2741—2746 |
| [34] | Corradini R., Paganuzzi C., Marchelli R., Pagliari S., Dossena A., Duchateau A., J. Inclusion Phenom. Macrocyclic Chem.,2007, 57, 625—630 |
| [35] | Richard G. I., Marwani H. M., Jiang S., Fakayode S. O., Lowry M., Strongin R. M., Warner I. M., Appl. Spectrosc., 2008, 62, 476—480 |
| [36] | Wang H., Chan W. H., Lee A. W. M., Org. Biomol. Chem.,2008, 6, 929—934 |
| [37] | Kantharaju, Babu V.V. S., Indian J. Chem.-Sec. B, 2006, 45,1942—1944 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||