

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (11): 2092.doi: 10.7503/cjcu20160437
• Polymer Chemistry • Previous Articles Next Articles
QIAN Liwei1,*( ), LI Ji2, SONG Wenqi2, HU Xiaoling2, GUAN Ping2
), LI Ji2, SONG Wenqi2, HU Xiaoling2, GUAN Ping2
Received:2016-06-17
Online:2016-11-10
Published:2016-09-20
Contact:
QIAN Liwei
E-mail:qianliwei@mail.nwpu.edu.cn
Supported by:CLC Number:
TrendMD:
QIAN Liwei, LI Ji, SONG Wenqi, HU Xiaoling, GUAN Ping. Utilizing Macromolecular Chain as Functional Monomer and Crosslinker to Imprint BSA with Preserving the Structural Integrity of Template†[J]. Chem. J. Chinese Universities, 2016, 37(11): 2092.
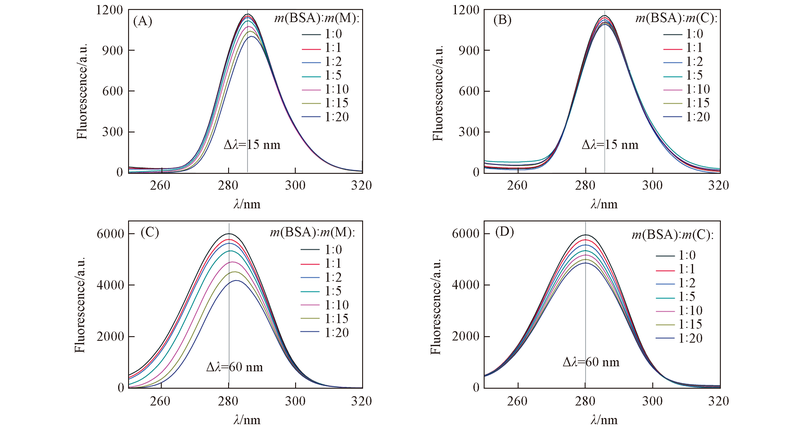
Fig.6 Synchronous fluorescence spectra of BSA influenced by micromolecular monomers(A, C) or macromolecular chains(B, D) (A), (B) Δλ =15 nm; (C), (D) Δλ =60 nm. M and C are represented micromolecular monomers and macromolecular chain, respectively.
| Sample | Km/(mL·mg-1) | Qm/(mg·g-1) | R2 |
|---|---|---|---|
| MIH-C-BSA | 2.79 | 91.7 | 0.9929 |
| NIH-C-BSA | 0.96 | 52.6 | 0.9926 |
| MIH-M-BSA | 1.65 | 78.7 | 0.9969 |
| NIH-M-BSA | 0.88 | 64.1 | 0.9829 |
Table 1 Theoretical maximum capacity(Qm) and Langmuir adsorption equilibrium constant(Km) from the Langmuir model
| Sample | Km/(mL·mg-1) | Qm/(mg·g-1) | R2 |
|---|---|---|---|
| MIH-C-BSA | 2.79 | 91.7 | 0.9929 |
| NIH-C-BSA | 0.96 | 52.6 | 0.9926 |
| MIH-M-BSA | 1.65 | 78.7 | 0.9969 |
| NIH-M-BSA | 0.88 | 64.1 | 0.9829 |
| Protein | Q(MIH-C-BSA)/ (mg·mg-1) | Q(NIH-C-BSA)/ (mg·mg-1) | IFa | βa | Q(MIH-M-BSA)/ (mg·mg-1) | Q(NIH-M-BSA)/ (mg·mg-1) | IFb | βb |
|---|---|---|---|---|---|---|---|---|
| BSA | 67.6 | 25.5 | 2.65 | 49.2 | 30.1 | 1.63 | ||
| OVA | 19.8 | 28.9 | 0.68 | 3.86 | 25.7 | 28.2 | 0.91 | 1.79 |
| Hb | 16.3 | 27.8 | 0.58 | 4.52 | 27.9 | 26.2 | 1.06 | 1.53 |
| Lyz | 6.7 | 13.1 | 0.51 | 5.18 | 9.2 | 13.4 | 0.68 | 2.38 |
Table 2 Selective adsorption experiments for MIH and NIH
| Protein | Q(MIH-C-BSA)/ (mg·mg-1) | Q(NIH-C-BSA)/ (mg·mg-1) | IFa | βa | Q(MIH-M-BSA)/ (mg·mg-1) | Q(NIH-M-BSA)/ (mg·mg-1) | IFb | βb |
|---|---|---|---|---|---|---|---|---|
| BSA | 67.6 | 25.5 | 2.65 | 49.2 | 30.1 | 1.63 | ||
| OVA | 19.8 | 28.9 | 0.68 | 3.86 | 25.7 | 28.2 | 0.91 | 1.79 |
| Hb | 16.3 | 27.8 | 0.58 | 4.52 | 27.9 | 26.2 | 1.06 | 1.53 |
| Lyz | 6.7 | 13.1 | 0.51 | 5.18 | 9.2 | 13.4 | 0.68 | 2.38 |
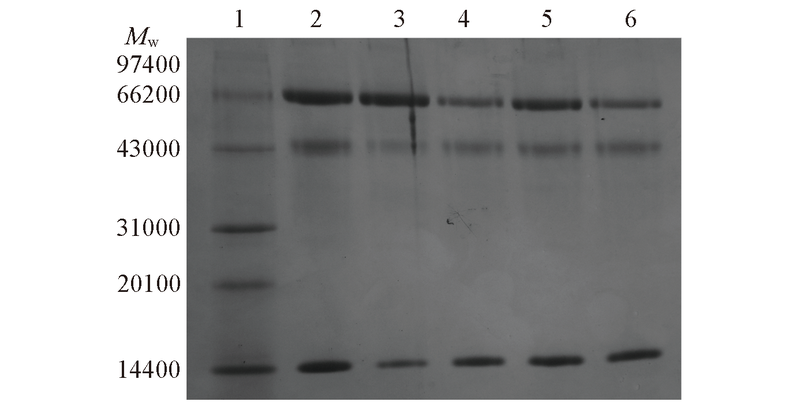
Fig.10 SDS-PAGE analysis of competitive adsorption of MIH-C-BSA and MIH-M-BSALane 1: protein molecular weight marker; lane 2: the mixture solution of BSA, OVA and Lyz with each concentration of 1.0 mg/mL; lane 3: the protein eluted from MIH-C-BSA; lane 4: the protein eluted from NIH-C-BSA; lane 5: the protein eluted from MIH-M-BSA; lane 6: the protein eluted from NIH-M-BSA.
| [1] | Chen L., X. , Wang X., Y. , Lu W., X. , Wu X., Q. , Li J., H. , Chemical Society Reviews, 2016, 45( 8), 2137- 2211 |
| [2] | Chen L., X. , Xu S., F. , Li J., H. , Chemical Society Reviews, 2011, 40( 5), 2922- 2942 |
| [3] | Zhang, Z. , Li J., H. , Fu J., Q. , Chen L., X. , RSC Advances, 2014, 4( 40), 20677- 20685 |
| [4] | Kryscio D., R. , Fleming M., Q. , Peppas N., A. , Macromolecular Bioscience, 2012, 12( 8), 1137- 1144 |
| [5] | Kryscio D., R. , Fleming M., Q. , Peppas N., A. , Biomedical Microdevices, 2012, 14( 4), 679- 687 |
| [6] | Qian L., W. , Hu X., L. , Guan, P. , Wang, D. , Li, J. , Du C., B. , Song R., Y. , RSC Advances, 2015, 5( 73), 59062- 59069 |
| [7] | Qian L., W. , Hu X., L. , Guan, P. , Wang, D. , Li, J. , Du C., B. , Song R., Y. , Wang C., L. , Song W., Q. , Analytica Chimica Acta, 2015, 884, 97- 105 |
| [8] | He, W. , Li, Y. , Xue, C. , Hu, Z. , Chen, X. , Sheng, F. , Bioorganic Medicinal Chemistry, 2005, 13( 5), 1837- 1845 |
| [9] | Kamat B., P. , Journal of Pharmaceutical and Biomedical Analysis, 2005, 39( 5), 1046- 1050 |
| [10] | Roach, P. , Farrar, D. , Perry C., C. , Journal of the American Chemical Society, 2005, 127( 22), 8168- 8173 |
| [11] | Hu, Z. , Tong, C. , Analytica Chimica Acta, 2007, 587( 2), 187- 193 |
| [12] | Pan, X. , Liu, R. , Qin, P. , Wang, L. , Zhao, X. , Journal of Luminescence, 2010, 130( 4), 611- 617 |
| [13] | Zhang, G. , Hu, X. , Zhao, N. , Li, W. , He, L. , Pesticide Biochemistry and Physiology, 2010, 98( 2), 206- 212 |
| [14] | Qin, L. , He X., W. , Zhang, W. , Li W., Y. , Zhang Y., K. , Analytical Chemistry, 2009, 81( 17), 7206- 7216 |
| [15] | Hawkins D., M. , Stevenson, D. , Reddy S., M. , Analytica Chimica Acta, 2005, 542, 61- 65 |
| [16] | Bi, W. , Tian, M. , Row K., H. , Journal of Chromatography A, 2012, 1232, 37- 42 |
| [17] | Fan J., P. , Tian Z., Y. , Tong, S. , Zhang X., H. , Xie Y., L. , Xu, R. , Qin, Y. , Li, L. , Zhang J., H. , Ouyang X., K. , Food Chemistry, 2013, 141( 4), 3578- 3585 |
| [18] | Bossi, A. , Bonini, F. , Turner A. P., F. , Piletsky S., A. , Biosensors and Bioelectronics, 2007, 22( 6), 1131- 1137 |
| [19] | Hiratani, H. , Macromolecular Bioscience, 2005, 5( 8), 728- 733 |
| [20] | Reddy S., M. , Phan Q., T. , El-Sharif, H. , Govada, L. , Stevenson, D. , Chayen N., E. , Biomacromolecules, 2012, 13( 12), 3959- 3965 |
| [21] | Tsermentseli S., K. , Manesiotis, P. , Assimopoulou A., N. , Papageorgiou V., P. , Journal of Chromatography A, 2013, 1315( 20), 15- 20 |
| [1] | DU Shanshan, LI Yang, GUO Lei, LI Pengyu, CHAI Zhilong, WANG Tao, QUAN Dongqin, HE Junlin. Modification of Aptamer TBA with Extra Functional Groups and the Biological Activities† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2445. |
| [2] | HE Xiaoqin, HE Junlin, XU Hua, GUO Lei, XIE Jianwei. Active Conformation of DNA Aptamer Against Recombinant Human Erythropoietin-α† [J]. Chem. J. Chinese Universities, 2018, 39(1): 48. |
| [3] | XU Lanlan, ZHAO Qiqi, YU He, WANG Jingchen, WANG Huijun, YANG Qin, ZHU Huajie, LI Yan. Absolute Configuration Determination of One New Compound Trichoderol A from Trichoderma sp. Fungus† [J]. Chem. J. Chinese Universities, 2016, 37(11): 1972. |
| [4] | ZHU Huajie, LIU Li, YANG Qin. Vibrational Circular Dichroism in Study of Stereochemistry of Chiral β-Biscarboline with N—O Functional Group [J]. Chem. J. Chinese Universities, 2015, 36(8): 1559. |
| [5] | JI Yannan, HE Ping, LI Jinliang, GUO Xiujie, WANG Xiangfen, YU He, SHEN Shigang, ZHU Huajie. Stereochemistry of Hydroxyscytalone Isolated from Penicillium Oxalicum Using VCD, ECD and OR Methods† [J]. Chem. J. Chinese Universities, 2014, 35(4): 746. |
| [6] | CHI Jia-Shu, SANG Yuan-Mei, YAN Li-Kai, SU Zhong-Min. Theoretical Study on Electronic Structure and Optical Activity of Chiral Polyoxometalates Modified by Amino Acids [J]. Chem. J. Chinese Universities, 2013, 34(10): 2370. |
| [7] | ZHANG Yue-Hong, JIANG Teng, ZHAO Xue-Zhou, ZHOU Ping. Inhibitory Effect of Curcumin on Metal Ions-induced Conformational Transition of Silk Fibroin and Its Mechanism [J]. Chem. J. Chinese Universities, 2013, 34(1): 225. |
| [8] | TANG Yu-Lin, GAO Zhan, XU Hong, HE Jian-Zhi, DONG Yi-Han, ZHENG Yi-Zhi. Interaction of Copper(II) Ion with SALI3-2 [J]. Chem. J. Chinese Universities, 2013, 34(1): 128. |
| [9] | SUN Xiu-Yu, LI Zong-Mu, XU Fa-Qiang, CHENG Ying-Zhi, XUE Li. X-Ray Magnetic Circular Dichroism Study on the Fe-group Magnetic Films by Electrodeposition [J]. Chem. J. Chinese Universities, 2012, 33(12): 2628. |
| [10] | WENG Shao-Huang, ZHOU Jian-Zhang, LIN Zhong-Hua, LIN Xin-Hua. Electrochemical Preparation of Chiral Polyaniline Nanofibers [J]. Chem. J. Chinese Universities, 2012, 33(11): 2501. |
| [11] | ZHAO Fang*, LIANG Hui, CHENG Hui, WANG Jun, ZHAO Wen-Hui. Interaction of Rhein-Cu(Ⅱ) Complexes with Bovine Serum Albumin [J]. Chem. J. Chinese Universities, 2011, 32(6): 1277. |
| [12] | WANG Qi, CHENG Xian, HE Rui, WANG Dong-Ni, LU Chang-Sheng*, MENG Qing-Jin. Different Assemblies of Monotonous Maleonitriledithiolate\|modified β-Cyclodextrins in Solution [J]. Chem. J. Chinese Universities, 2011, 32(3): 700. |
| [13] | CHEN Jun-Jie, YI Yu-Ting, YU Miao, XUE Shao-Long, XIONG Qian-Qian, HE Xiao-Meng, ZHANG Lian-Ru*. Inhibitation and Binding of Rugulosin with N-Hsp90 [J]. Chem. J. Chinese Universities, 2011, 32(1): 88. |
| [14] | NIE Xin-Hua, ZHANG Tao, LI Yuan*, ZHENG Xue-Fang*, CAO Hong-Yu, TANG Qian. Conformation Research of Shorted Glucagon-like Peptide-1 [J]. Chem. J. Chinese Universities, 2010, 31(7): 1337. |
| [15] | GUO Wei, WU Yong-Quan, ZHENG Lü-Yin, XU Li-Rong, FAN Xiao-Lin*. Interaction of Keyhole Limpet Hemocyanin with Niclosamide and Its Derivative [J]. Chem. J. Chinese Universities, 2009, 30(7): 1314. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||