

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (4): 654.doi: 10.7503/cjcu20141071
• Organic Chemistry • Previous Articles Next Articles
XIANG Junfeng, YI Pinggui*( ), YU Xianyong, CHEN Jian, HAO Yanlei, REN Zhiyong
), YU Xianyong, CHEN Jian, HAO Yanlei, REN Zhiyong
Received:2014-12-05
Online:2015-04-10
Published:2015-03-18
Contact:
YI Pinggui
E-mail:yipinggui@sohu.com
Supported by:CLC Number:
TrendMD:
XIANG Junfeng, YI Pinggui, YU Xianyong, CHEN Jian, HAO Yanlei, REN Zhiyong. Excited-state Proton Transfer of 2-(2-Hydroxyphenyl)benzothiazole in the Confined Nanocavity†[J]. Chem. J. Chinese Universities, 2015, 36(4): 654.
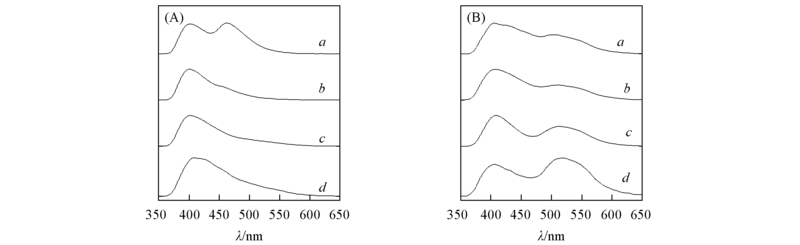
Fig.2 Fluorescence spectra of HBT in different solvents (A) a. DMSO, b. DMF, c. ethanol, d. THF; (B) a. hexamethylene, b. dioxane, c. dichloromethane, d. ethylacetate.
| V(CB7)/μL | λem/nm | Lifetime/ns | Amplitude(%) | Mean lifetime/ns | |||
|---|---|---|---|---|---|---|---|
| τ1 | τ2 | α1 | α2 | ||||
| 0 | 409 | 0.80 | 2.31 | 76.77 | 23.24 | 0.982 | 1.51 |
| 466 | 4.12 | 17.05 | 99.33 | 0.67 | 1.070 | 4.47 | |
| 10 | 409 | 0.82 | 2.50 | 83.22 | 16.78 | 1.014 | 1.46 |
| 466 | 2.66 | 4.29 | 9.98 | 90.02 | 1.081 | 4.19 | |
| 20 | 409 | 0.81 | 2.39 | 84.82 | 15.18 | 1.017 | 1.36 |
| 466 | 2.34 | 4.31 | 11.62 | 88.38 | 0.920 | 4.18 | |
| 30 | 409 | 0.86 | 2.49 | 88.10 | 11.90 | 1.046 | 1.32 |
| 466 | 1.85 | 4.22 | 6.22 | 93.78 | 0.986 | 4.15 | |
| 40 | 409 | 0.81 | 2.43 | 87.05 | 12.95 | 1.043 | 1.31 |
| 466 | 1.95 | 4.19 | 4.34 | 95.66 | 0.908 | 4.14 | |
| 50 | 409 | 0.77 | 2.33 | 85.32 | 14.68 | 0.944 | 1.30 |
| 466 | 1.91 | 4.20 | 5.32 | 94.68 | 0.991 | 4.14 | |
| 60 | 409 | 0.82 | 2.64 | 90.01 | 9.99 | 1.044 | 1.30 |
| 466 | 1.82 | 4.20 | 7.85 | 92.15 | 0.973 | 4.12 | |
| 70 | 409 | 0.85 | 2.74 | 91.64 | 8.36 | 1.103 | 1.28 |
| 466 | 1.44 | 4.15 | 4.44 | 95.56 | 1.078 | 4.11 | |
| 80 | 409 | 0.85 | 2.59 | 91.25 | 8.75 | 1.093 | 1.24 |
| 466 | 1.23 | 4.10 | 2.32 | 97.68 | 1.085 | 4.08 | |
Table 1 Fluorescence lifetime evaluated for HBT-CB7 complexes*
| V(CB7)/μL | λem/nm | Lifetime/ns | Amplitude(%) | Mean lifetime/ns | |||
|---|---|---|---|---|---|---|---|
| τ1 | τ2 | α1 | α2 | ||||
| 0 | 409 | 0.80 | 2.31 | 76.77 | 23.24 | 0.982 | 1.51 |
| 466 | 4.12 | 17.05 | 99.33 | 0.67 | 1.070 | 4.47 | |
| 10 | 409 | 0.82 | 2.50 | 83.22 | 16.78 | 1.014 | 1.46 |
| 466 | 2.66 | 4.29 | 9.98 | 90.02 | 1.081 | 4.19 | |
| 20 | 409 | 0.81 | 2.39 | 84.82 | 15.18 | 1.017 | 1.36 |
| 466 | 2.34 | 4.31 | 11.62 | 88.38 | 0.920 | 4.18 | |
| 30 | 409 | 0.86 | 2.49 | 88.10 | 11.90 | 1.046 | 1.32 |
| 466 | 1.85 | 4.22 | 6.22 | 93.78 | 0.986 | 4.15 | |
| 40 | 409 | 0.81 | 2.43 | 87.05 | 12.95 | 1.043 | 1.31 |
| 466 | 1.95 | 4.19 | 4.34 | 95.66 | 0.908 | 4.14 | |
| 50 | 409 | 0.77 | 2.33 | 85.32 | 14.68 | 0.944 | 1.30 |
| 466 | 1.91 | 4.20 | 5.32 | 94.68 | 0.991 | 4.14 | |
| 60 | 409 | 0.82 | 2.64 | 90.01 | 9.99 | 1.044 | 1.30 |
| 466 | 1.82 | 4.20 | 7.85 | 92.15 | 0.973 | 4.12 | |
| 70 | 409 | 0.85 | 2.74 | 91.64 | 8.36 | 1.103 | 1.28 |
| 466 | 1.44 | 4.15 | 4.44 | 95.56 | 1.078 | 4.11 | |
| 80 | 409 | 0.85 | 2.59 | 91.25 | 8.75 | 1.093 | 1.24 |
| 466 | 1.23 | 4.10 | 2.32 | 97.68 | 1.085 | 4.08 | |
| [1] | Weller A., Prog. React. Kinet., 1961, 1, 187—214 |
| [2] | Luque A. M., Mulder W. H., Calvente J. J., Cuesta A., Andreu R., Analytical Chemistry,2012, 84(13), 5778—5786 |
| [3] | Paul B. K., Samanta A., Guchhait N., Langmuir,2010, 26(5), 3214—3224 |
| [4] | Mutai T., Sawatani H., Shida T., Shono H., Araki K., J. Org. Chem., 2013, 78(6), 2482—2489 |
| [5] | Paul B. K., Guchhait N., J. Phys. Chem. B,2010, 114(39), 12528—12540 |
| [6] | Abderrazzak D., Francisco A. G., Acufia A. U., J. Phys. Chem., 1995, 99(1), 76—80 |
| [7] | Yi P. G., Liu J., Chen J., Yu X. Y., Li X. F., Zheng B. S., Tao H. W., Hao Y. L., Chem. J. Chinese Universities,2014, 35(6), 1219—1223 |
| (易平贵, 刘金, 陈建, 于贤勇, 李筱芳, 郑柏树, 陶洪文, 郝艳雷. 高等学校化学学报, 2014, 35(6), 1219—1223) | |
| [8] | Kim J., Jung I.S., Kim S. Y., Lee E., Kang J. K., Sakamoto S., Yamaguchi K., Kim K., J. Am. Chem. Soc., 2000, 122(3), 540—541 |
| [9] | Walker S., Oun R., Mcinnes F. J., Wheate N. J., Israel J. Chem., 2011, 51(5/6), 616—624 |
| [10] | Freeman W. A., Mock W. L., Shih N. Y., J. Am. Chem. Soc., 1981, 103(24), 7367—7368 |
| [11] | Shaikh M., Dutta C. S., Mohanty J., Bhasikuttan A. C., Nau W. M., Pal H., Chem. Eur. J., 2009, 15(45), 1236—12370 |
| [12] | Kaifer A. E., Acc. Chem. Res., 2014, 47(7), 2160—2167 |
| [13] | Basilio N., Laia C. A. T., Pina F., J. Phys. Chem. B,2015, 119(6), 2749—2757 |
| [14] | Hu Y., Wang J., Guo G. Y., Tao Z., Xue S. F., Zhu Q. J., Zhou Q. D., Chem. J. Chinese Universities,2013, 34(2), 375—380 |
| (黄英, 王娟, 郭改英, 陶朱, 薛赛凤, 祝黔江, 周清娣. 高等学校化学学报, 2013, 34(2), 375—380) | |
| [15] | Hu Y., Song G. X., Tang Q., Wang J., Tao Z., Xue S. F., Zhang J. X., Chem. J. Chinese Universities,2014, 35(6), 1224—1228 |
| (黄英, 宋桂先, 唐青, 王娟, 陶朱, 薛赛凤, 张建新. 高等学校化学学报, 2014, 35(6), 1224—1228) | |
| [16] | Lee J., Kim C. H., Joo T., J. Phys. Chem. A,2013, 117(7), 1400—1405 |
| [17] | Cohen B., Huppert D., Solntsev K. M., Tsfadia Y., Nachliel E., Gutman M., J. Am. Chem. Soc., 2002, 124(25), 7539—7547 |
| [18] | Corani A., Pezzella A., Pascher T., Gustavsson T., Markovitsi D., Huijser A., Ischia M. D.,Sundström V., J. Phys. Chem. Lett., 2013, 4(9), 1383—1388 |
| [19] | Bahrami K., Khodaei M. M., Naali F., J. Org. Chem., 2008, 73(17), 6835—6837 |
| [20] | Mohanty J., Bhasikuttan A. C., Nau W. M., Pal H., J. Phys. Chem. B,2004, 110(10), 5132—5138 |
| [21] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., KudinK. N., Staroverov V. N., Keith T., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision C.01, Gaussian Inc., Wallingford CT, 2010 |
| [22] | Aly S.M., Usman A., Alzayer M., Hamdi G. A., Alarousu E., Mohammed O. F.,J. Phys. Chem. B, 2014, DOI:10.1021/jp508777h |
| [23] | Polyakov N. E., Leshina T. V., Salakhutdinov N. F., Kispert L. D., J. Phys. Chem. B,2006, 110(13), 6991—6998 |
| [1] | ZHAO Dongxia, ZHANG Haixia, FENG Wenjuan, YANG Zhongzhi. Molecular Face Guiding the Proton Transfer Reactions of Hydroxyl Carbene and Its Derivatives [J]. Chem. J. Chinese Universities, 2021, 42(7): 2187. |
| [2] | ZENG Yonghui, YAN Tianying. Vibrational Density of States Analysis of Proton Hydration Structure [J]. Chem. J. Chinese Universities, 2021, 42(6): 1855. |
| [3] | TENG Yunyang, QU Zexing, ZHOU Zhongjun, HUANG Xuri. Theoretical Study on Photoinduced Stepwise Dearomatization of Benzenoid Arenes with Different States [J]. Chem. J. Chinese Universities, 2021, 42(3): 752. |
| [4] | WANG Ruxin, ZHAO Zhongjun, HE Feiyao, YUE Hanlu, DENG Fulong, LI Hong, LI Wenwen, DUAN Yixiang. Characteristic Analysis of C1—C3n-Aldehydes and n-Alcohols in Proton Transfer Reaction Time-of-flight Mass Spectrometry [J]. Chem. J. Chinese Universities, 2021, 42(12): 3632. |
| [5] | LI Kangming, LI Yansai, YI Yangjie, XU Leitao, YE Jiao, OU Xiaoming, LI Jianming, HU Aixi. Design, Synthesis and Biological Activity of 5-Pyrazole Carboxamides † [J]. Chem. J. Chinese Universities, 2020, 41(4): 716. |
| [6] | MA Xiangying, LIAO Yanjun, QIN Fanghong, YIN Yuanhao, HUANG Zaiyin, CHEN Qifeng. Study on the Photocatalytic Performance of Carbon Doped g-C3N4 Based on in situ Photomicrocalorimeter-fluorescence Spectrometry [J]. Chem. J. Chinese Universities, 2020, 41(11): 2526. |
| [7] | WEI Xin, DENG Yaoliang, ZHENG Xuming, ZHAO Yanying. Ground Structure and Excited State Proton Transfer Reaction of 2-Aminobenzothiazole [J]. Chem. J. Chinese Universities, 2019, 40(8): 1679. |
| [8] | LI Qing, YI Pinggui, TAO Hongwen, LI Yangyang, ZHANG Zhiyu, PENG Wenyu, LI Yuru. Solvent and Substituent Effects on Spectral Characteristics and Excited-state Intramolecular Proton Transfer of 2-(2-Aminophenyl) Benzothiazole† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1425. |
| [9] | BAI Lei,HUO Shuhui,CHEN Jing,LU Xiaoquan. Squaramide Fluorescence Probe for Chiral Recognition of α-Amino Acids† [J]. Chem. J. Chinese Universities, 2019, 40(1): 41. |
| [10] | ZHAO Bangtun, TAO Jingjing, CHEN Xiaoji, FU Huimin, ZHU Weimin. Synthesis, Structure and Electrochemistry of Tetrathiafulvalene Vinylogues Bearing Thienyl and Pyridyl Groups† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1449. |
| [11] | LI Yang, LI Zhiwen, ZHU Junfei, LIU Shihui, HE Junlin. Construction of Pyrenyl Pairs in dsDNA: Fluorescent Properties of Multiple Pyrenyl-attached dsDNAs Through 7-Substituted 8-Aza-7-deaza-2'-deoxyadenosine Analogues† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2206. |
| [12] | SUN Xiaoli, MENG Lingmin, JIN Mingxing, LI Jilai. Mechanism Research of Reactions of Metal Carbides and Carbenes Cation[M-X] +(M=Au, Ag, Cu; X=C, CH2) with Methane [J]. Chem. J. Chinese Universities, 2017, 38(8): 1406. |
| [13] | LI Xueying, CHEN Yanming, CUI Na, ZHANG Wanyu, WANG Zhiming. Excited State Intramolecular Proton Transfer of Salicylaldehyde Derivatives and Its Application in Fluorescent Probe Field† [J]. Chem. J. Chinese Universities, 2017, 38(3): 448. |
| [14] | ZHAO Bangtun, MA Shuxiu, TAO Jingjing, ZHU Weimin. Synthesis, Structures and Electrochemical Properties of Pyridine-based Tetrathiafulvalene Derivatives† [J]. Chem. J. Chinese Universities, 2017, 38(2): 193. |
| [15] | WAN Ting, LI Xingxing, HUANG Zaiyin, QIU Jiangyuan, ZUO Chen, TAN Xuecai. In-situ Photocatalytic Process of Rhodamine B over g-C3N4@Ag3PO4 Nanocomposites Based on Photomicrocalorimeter-fluorescence Spectrometry† [J]. Chem. J. Chinese Universities, 2017, 38(12): 2226. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||