

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (4): 733.doi: 10.7503/cjcu20180583
• Organic Chemistry • Previous Articles Next Articles
JIN Xin, JI Guoli, ZHAO Xiaoliang, LI Guoyun, YU Guangli*( )
)
Received:2018-08-20
Online:2019-03-11
Published:2019-03-11
Contact:
YU Guangli
E-mail:glyu@ouc.edu.cn
Supported by:CLC Number:
TrendMD:
JIN Xin,JI Guoli,ZHAO Xiaoliang,LI Guoyun,YU Guangli. Sulfated Arabinose from Codium fragile: Preparation and Extensive Structural Characterization†[J]. Chem. J. Chinese Universities, 2019, 40(4): 733.
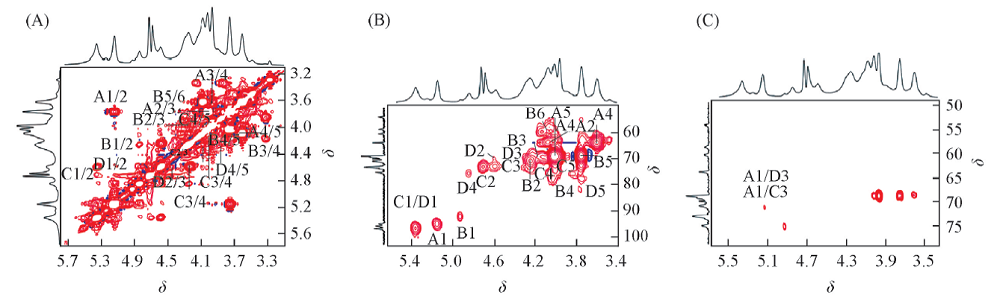
Fig.1 NMR spectra of polysaccharide CFP6 obtained from C. fragile in D2O at 25 ℃ (A) 1H-1H COSY spectrum; (B) 1H-13C HMQC spectrum; (C) 1H-13C HMBC spectrum.
| Residue | δ | |||||
|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | |
| Ara(A) | 5.15/95.05 | 3.77/69.09 | 4.01/68.88 | 3.61/70.63 | 4.08/63.66 | —— |
| Gal(B) | 4.84/91.97 | 4.24/70.63 | 4.13/69.15 | 3.96/71.14 | 3.75/69.00 | 4.14/61.53 |
| Ara2S(C) | 5.36/96.53 | 4.71/73.27 | 4.29/70.14 | 4.08/68.88 | 3.91/69.09 | —— |
| Ara4S(D) | 5.36/96.53 | 4.59/72.79 | 4.25/70.44 | 4.85/74.85 | 3.79/72.33 | —— |
Table 1 Chemical shifts(δ) for the resonances of glycosyl residues of CFP6 in 1H and 13C NMR spectra
| Residue | δ | |||||
|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | |
| Ara(A) | 5.15/95.05 | 3.77/69.09 | 4.01/68.88 | 3.61/70.63 | 4.08/63.66 | —— |
| Gal(B) | 4.84/91.97 | 4.24/70.63 | 4.13/69.15 | 3.96/71.14 | 3.75/69.00 | 4.14/61.53 |
| Ara2S(C) | 5.36/96.53 | 4.71/73.27 | 4.29/70.14 | 4.08/68.88 | 3.91/69.09 | —— |
| Ara4S(D) | 5.36/96.53 | 4.59/72.79 | 4.25/70.44 | 4.85/74.85 | 3.79/72.33 | —— |
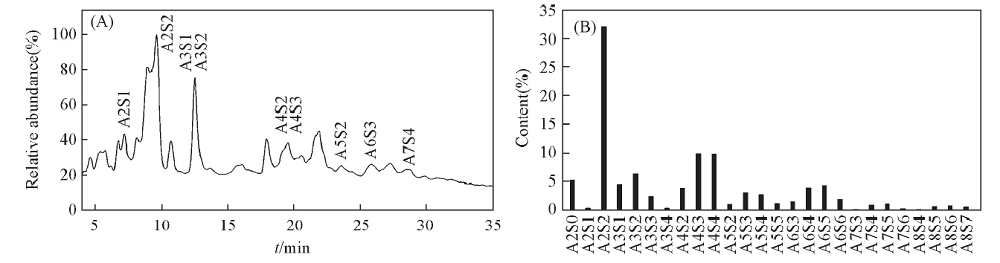
Fig.2 HILIC-FTMS profiling of CFP6 oligosaccharides generated by free radical depolymerization (A)Total ion chromatography of CFP6 oligosaccharides; (B) semiquantitative analysis of CFP6 oligosaccharides. A: Ara, S: sulfation, the following number represents the number of the previous residues.
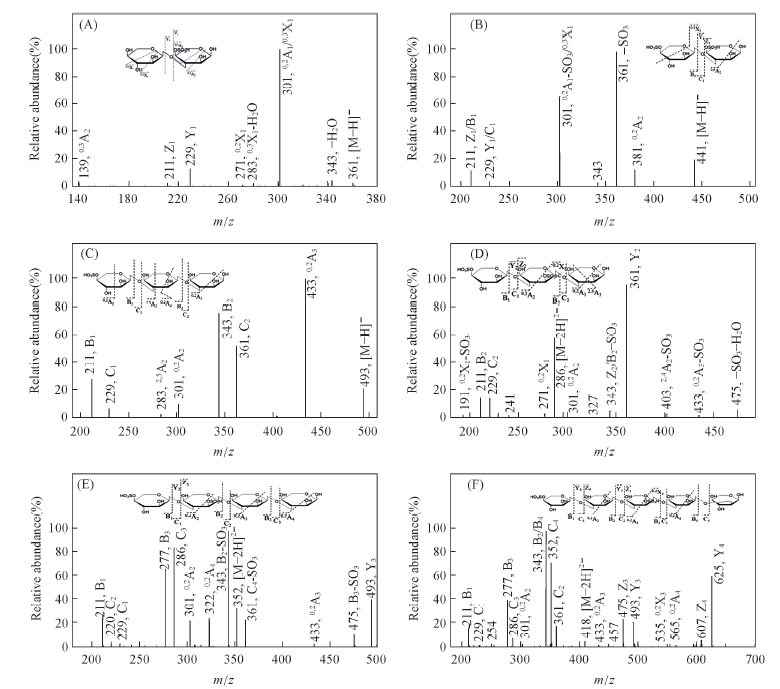
Fig.3 ESI-MS/MS spectra of CFP6 oligosaccharides and reduced oligosaccharides A2S1(A), A2S2(B), A3S1(C), A3S2(D), A4S2(E) and A5S2(F)(free acid form of oligosaccharides)
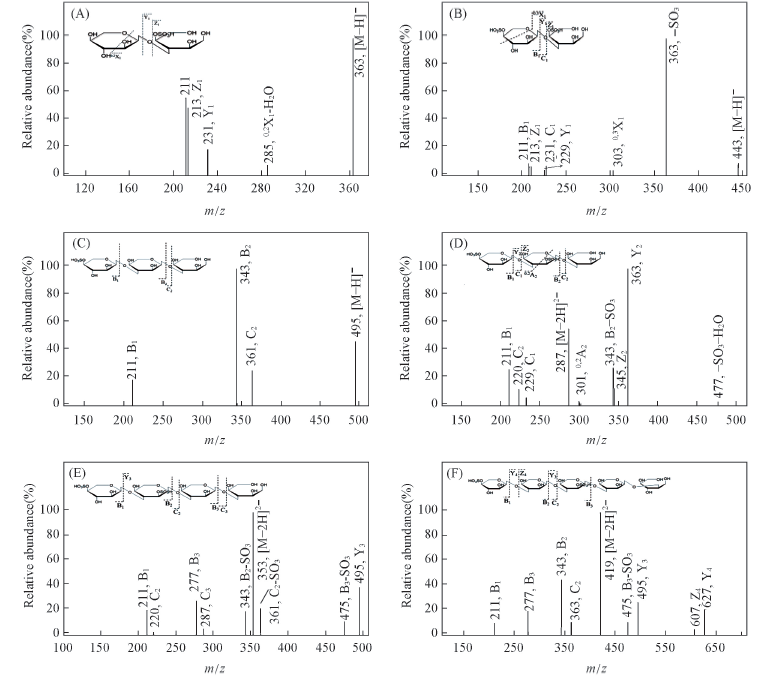
Fig.4 ESI-MS/MS spectra of CFP6 oligosaccharides and reduced oligosaccharides A2S1(A), A2S2(B), A3S1(C), A3S2(D), A4S2(E) and A5S2(F)(alditol form of reduced oligosaccharides)
| [1] | Shao H. Y., Ji H. W., Zhang C. H., Hong P. Z., Xiong H. P., Food Res.Dev.,2007, 28(10), 160—162 |
| (邵海艳, 吉宏武, 章超桦, 洪鹏志, 熊皓平. 食品研究与开发, 2007, 28(10), 160—162) | |
| [2] | Li N., Mao W. J., Yan M. X., Liu X., Xia Z., Wang S. Y., Xiao B., Chen C. L., Zhang L. F., Cao S. J., Carbohyd.Polym.,2015, 121, 175—182 |
| [3] | Shi Q., Wang A., Lu Z., Qin C., Hu J., Yin J., Carbohyd.Res.,2017, 453, 1—9 |
| [4] | Lu M. K., Lin T. Y., Chao C. H., Hu C. H., Hsu H. Y., Int. J. Biol.Macromol., 2017, 95, 1144—1152 |
| [5] | Nika K., Mulloy B., Carpenter B., Gibbs R., Eur. J.Phycol., 2003, 38(3), 257—264 |
| [6] | Kindness G., Long W. F., Williamson F. B., Brit. J.Pharmacol.,2012, 69(4), 675—677 |
| [7] | Tabarsa M., Han J. H., Kim C. Y., You S. G., J. Med.Food,2012, 15, 135—144 |
| [8] | Li H., Mao W., Zhang X., Qi X., Chen Y., Chen Y., Xu J., Zhao C., Hou Y., Yang Y., Li N., Wang C., Carbohyd.Polym., 2011, 85, 394—400 |
| [9] | Rihab B. A. K., Neila J., Faten H., Rim C., Imed J., Abdelfattah E., Tarak R., Kamel J., Lotfi F., Hafedh B., Karima B., Int. J. Biol.Macromol.,2017, 102, 119—129 |
| [10] | Yang J. L., Dong W. H., Chen H. Q., Kong F. D., Wang J., Mei W. L., Dai H. F., Chem. Res. Chinese Universities,2018, 34(3), 389—396 |
| [11] | Wang J. Q., Jiang W. Y., Liu Z. Y., Wang J. H., Fu T. Y., Wang Y. S., Chem. Res. Chinese Universities,2017, 33(5), 721—730 |
| [12] | Wang C., Lang Y., Li Q., Jin X., Li G., Yu G., Food Chem., 2018, 258, 231—236 |
| [13] | Nilsson J., Glycoconjugate J., 2016, 33(3), 261—272 |
| [14] | Wang T., Chu L., Li W., Lawson K., Apostol I., Eris T., Anal.Chem.,2017, 89(6), 3562—3567 |
| [15] | Ji G. L., Yu G. L., Wu J. D., Chin. J. Mar.Drug,2009, 28(3), 7—12 |
| (嵇国利, 于广利, 吴建东. 中国海洋药物, 2009, 28(3), 7—12 | |
| [16] | Dubois M., Gilles K. A., Hamilton J. K., Rebers P. T., Smith F., Anal.Chem.,1956, 28(3), 350—356 |
| [17] | Wu M., Xu S., Zhao J., Kang H., Ding H., Food Chem., 2010, 3, 716—723 |
| [18] | Chen S., XuJ., Xue C., Dong P., Sheng W., Yu G., Chai W., Glycoconjugate J., 2008, 25(5), 481—492 |
| [19] | Li Q., Cai C., Chang Y., Zhang F., Linhardt R. J., Xue C., Li G., Yu G., Carbohyd.Polym.,2018, 181, 1160—1168 |
| [20] | Estevez J. M., Fernandez P. V., Kasulin L., Glycobiology,2009, 19(3), 212—228 |
| [21] | Maria I. B., Ekaterina V., Alexander S., Carbohyd.Res., 2007, 342, 586—596 |
| [22] | Vaikundamoorthy R., Krishnamoorthy V., Vilwanathan R., Rajendran R., Int. J. Biol.Macromol.,2018, 111, 1229—1237 |
| [23] | Qi X., Mao W., Gao Y., Carbohyd.Polym.,2012, 90(4), 1804—1810 |
| [24] | Paula V. F., Irene Q., Alberto S. C., Julio J. C., Laercio P., Hugo V., José M. Estevez M., J. BioL.Chem., 2013, 288(1), 223—233 |
| [25] | Mao W., Fang F., Li H., Carbohyd.Polym.,2008, 74(4), 834—839 |
| [26] | Chen H. H., Zhao X., Luan X. H., Yu G. L., Chem. J. Chinese Universities,2015, 36(1), 1—8 |
| (陈欢欢, 赵峡, 栾晓红, 于广利. 高等学校化学学报, 2015, 36(1), 1—8) | |
| [27] | Ju H. L., Kanongnuch C., Peerapornpisa Y., Park W. J., You S. G.,Biosci. Biotech.Bioch.,2016, 80(5), 972—982 |
| [28] | Kiminori M., Yasushi M., Kanji H., Keisuke M., J. Appl.Phycol.(,2000, 121), 9—14 |
| [1] | BAO Han, LUO Jing, SHI Lianxin, XU Fujian, WANG Shutao. Fabricating Polysaccharide Micro-hydrogel via Superhydrophobic Pillar-structured Si Substrate † [J]. Chem. J. Chinese Universities, 2020, 41(7): 1484. |
| [2] | WANG Tingting, LI Yuan, YANG Lili, BAO Changhao, CHENG Han. In vivo Dynamic Detection of Aloe Polysaccharides Using Carbon Fiber Microelectrodes Modified with Gold Nanoparticles † [J]. Chem. J. Chinese Universities, 2020, 41(1): 87. |
| [3] | LING Yao, LIU Xuejing, HAO Haijing, HAO Xiaohui, BAI Libin, WU Yonggang. Synthesis and Properties of Water-soluble Glycosyl Fluorescent Polymer with Aggregation-induced Emission Effect† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1319. |
| [4] | GAO Yunxiao,HU Xiaolong,WANG Yuerong,JIANG Zhihong,ZHANG Hongyang,ZHANG Min,HU Ping. Primary Structural Analysis of Dendrobium officinale Polysaccharide† [J]. Chem. J. Chinese Universities, 2018, 39(5): 934. |
| [5] | ZHAO Qi, HE Wanying, DUAN Lijie, ZHANG Yu, YU Shuangjiang, GAO Guanghui. Fabrication and Characterization of Injectable Polysaccharide-polypeptide Hydrogel Based on Schiff’s Base† [J]. Chem. J. Chinese Universities, 2016, 37(9): 1750. |
| [6] | SHEN Yanhong, CHEN Linxiao, ZHANG Wenqing, XU Zhizhen, WAN Yanfei, ZHANG Li, XIA Wei. Structural Characterization of Mannatide† [J]. Chem. J. Chinese Universities, 2016, 37(6): 1088. |
| [7] | ZHI Zijian, ZOU Mingming, LI Shan, CHEN Jianle, YE Xingqian, CHEN Shiguo. Rheological and Structural Characterization of Pectin Polysaccharides from Citrus Pulp† [J]. Chem. J. Chinese Universities, 2016, 37(6): 1175. |
| [8] | LIU Yang, YIN Lu, GONG Guiping, PENG Yifang, HUANG Linjuan, WANG Zhongfu. Structural Characterization, Antioxidant Activity and Immunomodulatory Activity of the Polysaccharide LRLP3 from Leaves of Lycium ruthenicum Murra† [J]. Chem. J. Chinese Universities, 2016, 37(2): 261. |
| [9] | LIU Lei, LI Ke, HAO Xia, WANG Guizhen, QIN Xuemei, DU Guanhua, ZHANG Xiang. Extraction, Separation, Structural Analysis and Antioxidant Activity in vitro of Arabinoxylans(AX-Ⅰ-1) from the Residue of Astragalus Root† [J]. Chem. J. Chinese Universities, 2016, 37(12): 2168. |
| [10] | FENG Jilu, QI Junru, LIU Qianru. Fabrication of Soy Protein Isolate-soluble Soy Polysaccharide Core-shell Nanogels via Maillard Reaction and Self-assembly† [J]. Chem. J. Chinese Universities, 2016, 37(11): 1999. |
| [11] | GUAN Qingxiang, ZHANG Guangyuan, SUN Dandan, SUN Shilin, SUN Cheng, LIU Xin, HAN Bing. Investigation on Synthesis of Bletilla Striata Polysaccharides Amphiphilic Polymer and Drug-loaded Delivery System† [J]. Chem. J. Chinese Universities, 2016, 37(10): 1915. |
| [12] | YIN Junyi, LIU Xiaoying, NIE Shaoping, XIE Mingyong. Solution, Conformational Properties and Morphology of Psyllium Polysaccharide with Ferulic Acid Removal† [J]. Chem. J. Chinese Universities, 2016, 37(1): 43. |
| [13] | WANG Yiyu, LIU Jiawei, SIMA Zhenhua, SONG Houpan, LI Ruliu, CAI Jiazhong, CHEN Weiwen. Isolation, Structural Characterization and IEC-6 Cell Migration Activities of Polysaccharides From Atractylodes Macrocephala Koidz† [J]. Chem. J. Chinese Universities, 2015, 36(2): 299. |
| [14] | ZHANG Xia, ZHOU Hao, YANG Yuhong, HUANG Yufang, CHEN Xin. Oxidized Cellulose Enhanced Collagen Hydrogels† [J]. Chem. J. Chinese Universities, 2015, 36(10): 2040. |
| [15] | LI Xiaojun, JIANG Jiaye, SHI Songshan, LI Yuan, JIANG Yongbo, KE Yan, WANG Shunchun. Anti-complementary Activities of a (1→6) Linked Glucan from Korean Mondshood Root and Its Sulfated Derivatives† [J]. Chem. J. Chinese Universities, 2014, 35(7): 1423. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||