

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (8): 1460.doi: 10.7503/cjcu20160346
• Physical Chemistry • Previous Articles Next Articles
LI Lei, HUANG Cuiying, JIANG Xiaonan, GAO Xichan, WANG Changsheng*( )
)
Received:2016-05-16
Online:2016-07-19
Published:2016-07-19
Contact:
WANG Changsheng
E-mail:chwangcs@lnnu.edu.cn
Supported by:CLC Number:
TrendMD:
LI Lei,HUANG Cuiying,JIANG Xiaonan,GAO Xichan,WANG Changsheng. Ionic Hydrogen Bonding Between Arginine Side Chain and Nucleic Acid Bases†[J]. Chem. J. Chinese Universities, 2016, 37(8): 1460.
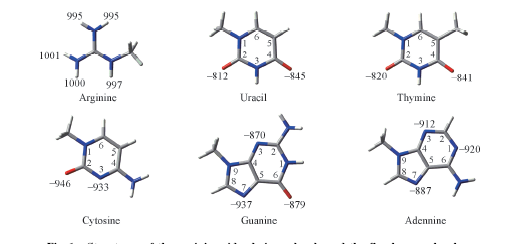
Fig.1 Structures of the arginine side chain molecule and the five base molecules The ΔH(kJ/mol) of the protonation and deprotonation reactions associated with the hydrogen bonding sites obtained at the MP2/aug-cc-pVTZ//MP2/aug-cc-pVDZ level were given in the corresponding sites.
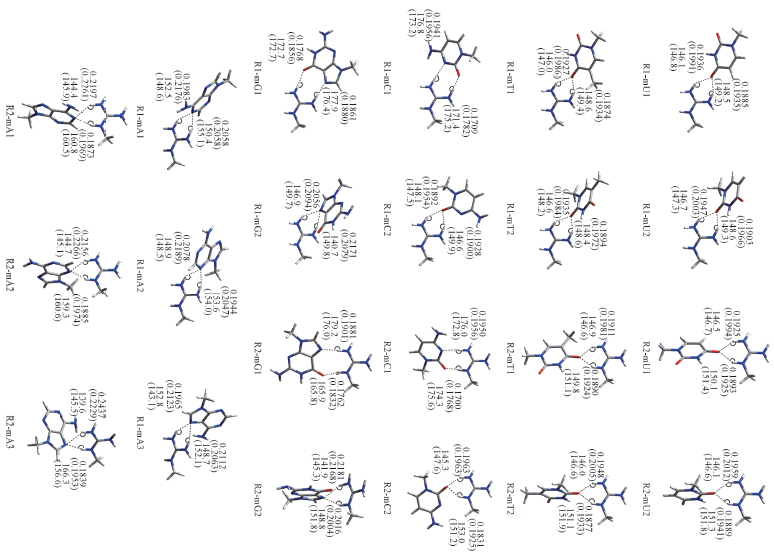
Fig.2 Optimal structures of 22 hydrogen-bonded complexes The hydrogen bond distances[RNH…X (nm, X=N or O)] and bond angles[∠NHX(°)] in gas phase and in water phase are given in the corresponding positions and in parentheses, respectively.
| Complex | Type | RNH…X/nm | Eb/(kJ·mol-1) | ΔE(2) / (kJ·mol-1) | ΣΔE(2) / (kJ·mol-1) | ρc/a.u. | Σρc/a.u. | qt/e |
|---|---|---|---|---|---|---|---|---|
| R1-mU1 | N—H…O | 0.1885 | -104.27(-39.70) | 63.76 | 109.16 | 0.0279 | 0.0522 | 0.0371 |
| N—H…O | 0.1936 | 45.40 | 0.0243 | |||||
| R1-mU2 | N—H…O | 0.1905 | -87.49(-37.91) | 48.91 | 85.14 | 0.0263 | 0.0498 | 0.0333 |
| N—H…O | 0.1947 | 36.23 | 0.0234 | |||||
| R2-mU1 | N—H…O | 0.1893 | -104.01(-40.77) | 59.66 | 111.08 | 0.0266 | 0.0522 | 0.0373 |
| N—H…O | 0.1925 | 51.42 | 0.0256 | |||||
| R2-mU2 | N—H…O | 0.1889 | -87.45(-39.35) | 56.07 | 90.71 | 0.0272 | 0.0501 | 0.0337 |
| N—H…O | 0.1959 | 34.64 | 0.0229 | |||||
| R1-mT1 | N—H…O | 0.1874 | -104.93(-42.06) | 70.58 | 117.48 | 0.0289 | 0.0538 | 0.0385 |
| N—H…O | 0.1927 | 46.90 | 0.0249 | |||||
| R1-mT2 | N—H…O | 0.1894 | -93.51(-39.80) | 51.00 | 89.24 | 0.0270 | 0.0512 | 0.0348 |
| N—H…O | 0.1935 | 38.24 | 0.0241 | |||||
| R2-mT1 | N—H…O | 0.1890 | -105.23(-43.05) | 62.51 | 123.51 | 0.0270 | 0.0539 | 0.0391 |
| N—H…O | 0.1911 | 61.00 | 0.0269 | |||||
| R2-mT2 | N—H…O | 0.1877 | -93.51(-41.03) | 58.41 | 94.81 | 0.0280 | 0.0515 | 0.0353 |
| N—H…O | 0.1948 | 36.40 | 0.0235 | |||||
| R1-mC1 | N—H…O | 0.1709 | -151.08(-62.45) | 156.06 | 245.05 | 0.0428 | 0.0729 | 0.0822 |
| N—H…N | 0.1941 | 88.99 | 0.0300 | |||||
| R1-mC2 | N—H…O | 0.1892 | -144.72(-51.31) | 61.46 | 115.18 | 0.0288 | 0.0546 | 0.0472 |
| N—H…O | 0.1928 | 53.72 | 0.0258 | |||||
| R2-mC1 | N—H…O | 0.1700 | -152.59(-63.58) | 172.05 | 258.32 | 0.0443 | 0.0738 | 0.0858 |
| N—H…N | 0.1950 | 86.27 | 0.0295 | |||||
| R2-mC2 | N—H…O | 0.1831 | -147.11(-55.19) | 96.65 | 137.11 | 0.0353 | 0.0577 | 0.0555 |
| N—H…O | 0.1963 | 40.46 | 0.0224 | |||||
| R1-mG1 | N—H…O | 0.1768 | -158.82(-59.90) | 123.34 | 245.55 | 0.0362 | 0.0716 | 0.0776 |
| N—H…N | 0.1861 | 122.21 | 0.0354 | |||||
| R1-mG2 | N—H…O | 0.2056 | -142.93(-46.98) | 47.03 | 74.90 | 0.0241 | 0.0436 | 0.0387 |
| N—H…O | 0.2171 | 27.87 | 0.0195 | |||||
| R2-mG1 | N—H…O | 0.1762 | -160.96(-61.35) | 133.68 | 249.45 | 0.0375 | 0.0713 | 0.0790 |
| N—H…N | 0.1881 | 115.77 | 0.0338 | |||||
| R2-mG2 | N—H…O | 0.2017 | -144.39(-48.66) | 59.54 | 86.07 | 0.0263 | 0.0454 | 0.0437 |
| N—H…O | 0.2181 | 26.53 | 0.0190 | |||||
| R1-mA1 | N—H…N | 0.1983 | -95.31(-39.73) | 69.37 | 105.90 | 0.0291 | 0.0535 | 0.0515 |
| N—H…N | 0.2058 | 36.53 | 0.0244 | |||||
| R1-mA2 | N—H…N | 0.1944 | -91.63(-40.76) | 75.02 | 110.75 | 0.0310 | 0.0545 | 0.0529 |
| N—H…N | 0.2078 | 35.73 | 0.0235 | |||||
| R1-mA3 | N—H…N | 0.1965 | -90.04(-42.83) | 81.46 | 105.69 | 0.0297 | 0.0507 | 0.0462 |
| N—H…N | 0.2112 | 24.23 | 0.0210 | |||||
| R2-mA1 | N—H…N | 0.1873 | -96.11(-43.43) | 116.69 | 134.39 | 0.0370 | 0.0555 | 0.0587 |
| N—H…N | 0.2197 | 17.70 | 0.0185 | |||||
| R2-mA2 | N—H…N | 0.1885 | -91.59(-41.37) | 103.68 | 127.53 | 0.0353 | 0.0554 | 0.0574 |
| N—H…N | 0.2156 | 23.85 | 0.0200 | |||||
| R2-mA3 | N—H…N | 0.1839 | -90.25(-43.52) | 138.49 | 140.37 | 0.0382 | 0.0493 | 0.0563 |
| N—H…N | 0.2437 | 1.88 | 0.0111 |
Table 1 Hydrogen bond distances(RNH…X), binding energies(Eb), the second-order stabilization energies(ΔE(2)), the electron densities(ρc) at the hydrogen bond critical points, and the charge transfer(qt) between two molecules for the gas phase structures*
| Complex | Type | RNH…X/nm | Eb/(kJ·mol-1) | ΔE(2) / (kJ·mol-1) | ΣΔE(2) / (kJ·mol-1) | ρc/a.u. | Σρc/a.u. | qt/e |
|---|---|---|---|---|---|---|---|---|
| R1-mU1 | N—H…O | 0.1885 | -104.27(-39.70) | 63.76 | 109.16 | 0.0279 | 0.0522 | 0.0371 |
| N—H…O | 0.1936 | 45.40 | 0.0243 | |||||
| R1-mU2 | N—H…O | 0.1905 | -87.49(-37.91) | 48.91 | 85.14 | 0.0263 | 0.0498 | 0.0333 |
| N—H…O | 0.1947 | 36.23 | 0.0234 | |||||
| R2-mU1 | N—H…O | 0.1893 | -104.01(-40.77) | 59.66 | 111.08 | 0.0266 | 0.0522 | 0.0373 |
| N—H…O | 0.1925 | 51.42 | 0.0256 | |||||
| R2-mU2 | N—H…O | 0.1889 | -87.45(-39.35) | 56.07 | 90.71 | 0.0272 | 0.0501 | 0.0337 |
| N—H…O | 0.1959 | 34.64 | 0.0229 | |||||
| R1-mT1 | N—H…O | 0.1874 | -104.93(-42.06) | 70.58 | 117.48 | 0.0289 | 0.0538 | 0.0385 |
| N—H…O | 0.1927 | 46.90 | 0.0249 | |||||
| R1-mT2 | N—H…O | 0.1894 | -93.51(-39.80) | 51.00 | 89.24 | 0.0270 | 0.0512 | 0.0348 |
| N—H…O | 0.1935 | 38.24 | 0.0241 | |||||
| R2-mT1 | N—H…O | 0.1890 | -105.23(-43.05) | 62.51 | 123.51 | 0.0270 | 0.0539 | 0.0391 |
| N—H…O | 0.1911 | 61.00 | 0.0269 | |||||
| R2-mT2 | N—H…O | 0.1877 | -93.51(-41.03) | 58.41 | 94.81 | 0.0280 | 0.0515 | 0.0353 |
| N—H…O | 0.1948 | 36.40 | 0.0235 | |||||
| R1-mC1 | N—H…O | 0.1709 | -151.08(-62.45) | 156.06 | 245.05 | 0.0428 | 0.0729 | 0.0822 |
| N—H…N | 0.1941 | 88.99 | 0.0300 | |||||
| R1-mC2 | N—H…O | 0.1892 | -144.72(-51.31) | 61.46 | 115.18 | 0.0288 | 0.0546 | 0.0472 |
| N—H…O | 0.1928 | 53.72 | 0.0258 | |||||
| R2-mC1 | N—H…O | 0.1700 | -152.59(-63.58) | 172.05 | 258.32 | 0.0443 | 0.0738 | 0.0858 |
| N—H…N | 0.1950 | 86.27 | 0.0295 | |||||
| R2-mC2 | N—H…O | 0.1831 | -147.11(-55.19) | 96.65 | 137.11 | 0.0353 | 0.0577 | 0.0555 |
| N—H…O | 0.1963 | 40.46 | 0.0224 | |||||
| R1-mG1 | N—H…O | 0.1768 | -158.82(-59.90) | 123.34 | 245.55 | 0.0362 | 0.0716 | 0.0776 |
| N—H…N | 0.1861 | 122.21 | 0.0354 | |||||
| R1-mG2 | N—H…O | 0.2056 | -142.93(-46.98) | 47.03 | 74.90 | 0.0241 | 0.0436 | 0.0387 |
| N—H…O | 0.2171 | 27.87 | 0.0195 | |||||
| R2-mG1 | N—H…O | 0.1762 | -160.96(-61.35) | 133.68 | 249.45 | 0.0375 | 0.0713 | 0.0790 |
| N—H…N | 0.1881 | 115.77 | 0.0338 | |||||
| R2-mG2 | N—H…O | 0.2017 | -144.39(-48.66) | 59.54 | 86.07 | 0.0263 | 0.0454 | 0.0437 |
| N—H…O | 0.2181 | 26.53 | 0.0190 | |||||
| R1-mA1 | N—H…N | 0.1983 | -95.31(-39.73) | 69.37 | 105.90 | 0.0291 | 0.0535 | 0.0515 |
| N—H…N | 0.2058 | 36.53 | 0.0244 | |||||
| R1-mA2 | N—H…N | 0.1944 | -91.63(-40.76) | 75.02 | 110.75 | 0.0310 | 0.0545 | 0.0529 |
| N—H…N | 0.2078 | 35.73 | 0.0235 | |||||
| R1-mA3 | N—H…N | 0.1965 | -90.04(-42.83) | 81.46 | 105.69 | 0.0297 | 0.0507 | 0.0462 |
| N—H…N | 0.2112 | 24.23 | 0.0210 | |||||
| R2-mA1 | N—H…N | 0.1873 | -96.11(-43.43) | 116.69 | 134.39 | 0.0370 | 0.0555 | 0.0587 |
| N—H…N | 0.2197 | 17.70 | 0.0185 | |||||
| R2-mA2 | N—H…N | 0.1885 | -91.59(-41.37) | 103.68 | 127.53 | 0.0353 | 0.0554 | 0.0574 |
| N—H…N | 0.2156 | 23.85 | 0.0200 | |||||
| R2-mA3 | N—H…N | 0.1839 | -90.25(-43.52) | 138.49 | 140.37 | 0.0382 | 0.0493 | 0.0563 |
| N—H…N | 0.2437 | 1.88 | 0.0111 |
| [1] |
Meot-Ner, M. , Chem. Rev., 2005, 105( 1), 213- 284
doi: 10.1002/chin.200515284 URL pmid: 15729772 |
| [2] |
Meot-Ner, M. , Chem. Rev., 2012, 112( 1), 22- 103
doi: 10.1021/cr200430n URL pmid: 22873941 |
| [3] | Vá, zquez M. E. , Caamañ, o A. M. , Mascareñ, as J. L. , Chem. Soc. Rev., 2003, 32( 6), 338- 349 |
| [4] |
Shulman-Peleg, A. , Shatsky, M. , Nussinov, R. , Wolfson H., J. , J. Mol. Biol., 2008, 379( 2), 299- 316
doi: 10.1016/j.jmb.2008.03.043 URL pmid: 18452949 |
| [5] |
Antson A., A. , Dodson E., J. , Dodson, G. , Greaves R., B. , Chen X., P. , Gollnick, P. , Nature, 1999, 401, 235- 242
doi: 10.1038/45730 URL pmid: 10499579 |
| [6] |
Gu, J. , Wang, J. , Leszczynski, J. , J. Phys. Chem. B, 2006, 110( 27), 13590- 13596
doi: 10.1021/jp061360x URL pmid: 16821886 |
| [7] |
Wang, P. , Zhang J. Z., H. , J. Phys. Chem. B, 2010, 114( 40), 12958- 129640
doi: 10.1021/jp1030224 URL pmid: 20860351 |
| [8] |
Li, Y. , Jiang X., N. , Wang C., S. , J. Comput. Chem., 2011, 32( 5), 953- 966
doi: 10.1002/jcc.21680 URL pmid: 20949514 |
| [9] | Li, Y. , Wang C., S. , J. Comput. Chem., 2011, 32( 13), 2765- 2773 |
| [10] | Huang C., Y. , Li, Y. , Wang C., S. , Sci. China Chem., 2013, 56( 2), 238- 248 |
| [11] |
刘朋, 李书实, 王长生. 高等学校化学学报, 2014, 35( 1), 154- 160
doi: 10.7503/cjcu20130707 |
|
Liu, P. , Li S., S. , Wang C., S. , Chem. J. Chinese Universities, 2014, 35( 1), 154- 160
doi: 10.7503/cjcu20130707 |
|
| [12] |
Seeman N., C. , Rosenberg J., M. , Rich, A. , Proc. Natl. Acad. Sci. USA, 1976, 73( 3), 804- 808
doi: 10.1073/pnas.73.3.804 URL pmid: 1062791 |
| [13] |
Lehmann M., S. , Verbist J., J. , Hamilton W., C. , Koetzle T., F. , J. Chem. Soc. Perk Trans. II, 1973, 133-137
doi: 10.1039/p29730000133 URL |
| [14] | Luscombe N., M. , Laskowski R., A. , Thornton J., M. , Nucl. Acids Res., 2001, 29( 13), 2860- 2874 |
| [15] |
Cheng A., C. , Chen W., W. , Fuhrmann C., N. , Frankel A., D. , J. Mol. Biol., 2003, 327( 4), 781- 796
doi: 10.1016/S0022-2836(03)00091-3 URL pmid: 12654263 |
| [16] |
Rozas, I. , Alkortab, I. , Elguerob, J. , Org. Biomol. Chem., 2005, 3( 2), 366- 371
doi: 10.1007/s11224-005-1089-9 URL |
| [17] |
Lejeune, D. , Delsaux, N. , Charloteaux, B. , Thomas, A. , Brasseur, R. , Proteins:, Struct. , Funct., Bioinform., 2005, 61( 2), 258- 271
doi: 10.1002/prot.20607 URL pmid: 16121397 |
| [18] | Hao J., J. , Li S., S. , Jiang X., N. , Li X., L. , Wang C., S. , Theor. Chem. Acc., 2014, 133, 1516- 1527 |
| [19] |
Scheiner, S. , J. Phys. Chem. B, 2005, 109( 33), 16132- 16141
doi: 10.1021/jp053416d URL pmid: 16853050 |
| [20] |
Scheiner, S. , J. Phys. Chem. B, 2006, 110( 37), 18670- 18679
doi: 10.1021/jp063225q URL pmid: 16970498 |
| [21] |
Hao J., J. , Wang C., S. , RSC Adv., 2015, 5( 9), 6452- 6461
doi: 10.1039/c4ra16359a URL |
| [22] |
Riley K., E. , Hobza, P. , J. Phys. Chem. A, 2007, 111( 33), 8257- 8263
doi: 10.1021/jp073358r URL pmid: 17649987 |
| [23] |
Li S., S. , Huang C., Y. , Hao J., J. , Wang C., S. , J. Comput. Chem., 2014, 35( 6), 415- 426
doi: 10.1002/jcc.23473 URL pmid: 24497309 |
| [24] |
Nagy P., I. , Erhardt P., W. , J. Phys. Chem. A, 2008, 112( 18), 4342- 4354
doi: 10.1021/jp7108847 URL pmid: 18373368 |
| [25] |
Hanus M. ,
doi: 10.1016/0304-3835(86)90172-2 URL pmid: 3004720 |
| [26] |
Steindal A., H. , Ruud, K. , Frediani, L. , Aidas, K. , Kongsted, J. , J. Phys. Chem. B, 2011, 115( 12), 3027- 3037
doi: 10.1021/jp1101913 URL pmid: 21391548 |
| [27] | Bader R. F., W. , Chem. Rev., 1991, 91( 5), 893- 926 |
| [28] |
Reed A., E. , Weinhold, F. , J. Chem. Phys., 1983, 78( 6), 4066- 4073
doi: 10.1063/1.445134 URL |
| [29] | Frisch M., J. , Trucks G., W. , Schlegel H., B. , Scuseria G., E. , Robb M., A. , Cheeseman J., R. , Scalmani, G. , Barone, V. , Mennucci, B. , Petersson G., A. , Nakatsuji, H. , Caricato, M. , Li, X. , Hratchian H., P. , Izmaylov A., F. , Bloino, J. , Zheng, G. , Sonnenberg J., L. , Hada, M. , Ehara, M. , Toyota, K. , Fukuda, R. , Hasegawa, J. , Ishida, M. , Nakajima, T. , Honda, Y. , Kitao, O. , Nakai, H. , Vreven, T. , Montgomery J. A., Jr. , Peralta J., E. , Ogliaor, F. , Bearpark, M. , Heyd J., J. , Brothers, E. , Kudin K., N. , Staroverov V., N. , Keith, T. , Kobayashi, R. , Normand, J. , Raghavachari, K. , Rendell, A. , Burant J., C. , Iyengar S., S. , Tomasi, J. , Cossi, M. , Rega, N. , Millam J., M. , Klene, M. , Knox J., E. , Cross J., B. , Bakken, V. , Adamo, C. , Jaramillo, J. , Gomperts, R. , Stratmann R., E. , Yazyev, O. , Austin A., J. , Cammi, R. , Pomelli, C. , Ochterski J., W. , Martin R., L. , Morokuma, K. , Zakrzewski V., G. , Voth G., A. , Salvador, P. , Dannenberg J., J. , Dapprich, S. , Daniels A., D. , Farkas, O. , Foresman J., B. , Ortiz J., V. , Cioslowski, J. , Fox D., J. , Gaussian, 09 , Revision D., 01 , Gaussian, Inc. , Wallingford, CT, 2013 |
| [30] |
Biegler-Kö, nig F. , Schö, nbohm J. , Bayles, D. , J. Comput. Chem., 2001, 22( 5), 545- 559
doi: 10.1002/1096-987X(20010415)22:5<545::AID-JCC1027>3.0.CO;2-Y URL |
| [31] |
张敏, 郑艳萍, 姜笑楠, 王长生. 物理化学学报, 2010, 26( 3), 735- 739
doi: 10.3866/PKU.WHXB20100235 |
|
Zhang, M. , Zheng Y., P. , Jiang X., N. , Wang C., S. , Acta Phys.-Chim. Sin., 2010, 26( 3), 735- 739
doi: 10.3866/PKU.WHXB20100235 |
|
| [32] |
Caramori G., F. , Galembeck S., E. , J. Phys. Chem. A, 2007, 111( 9), 1705- 1712
doi: 10.1021/jp066863h URL pmid: 17295458 |
| [33] |
Zhou P., P. , Qiu W., Y. , Chem. Phys. Chem., 2009, 10( 11), 1847- 1858
doi: 10.1021/jp9035452 URL pmid: 19715282 |
| [34] |
Sun, L. , Cukier R., I. , Bu, Y. , J. Phys. Chem. B, 2007, 111( 7), 1802- 1808
doi: 10.1021/jp063645f URL pmid: 17266349 |
| [35] |
刘畅, 于歌, 黄翠英, 王长生. 化学学报, 2015, 73( 4), 357- 365
doi: 10.6023/A14120869 |
|
Liu, C. , Yu, G. , Huang C., Y. , Wang C., S. , Acta Chim. Sinica, 2015, 73( 4), 357- 365
doi: 10.6023/A14120869 |
| [1] | LI Jinxing,XING Xiaofeng,QI Zhongnan,AI Hongqi. Effects of Three New Modified Molecules on the Structural Stability of Different Aβ42 Fibers† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2230. |
| [2] | LI Lei, LI Shushi, WANG Changsheng. Theoretical Studies on Noncovalent Interactions Between Charged Histidine Side Chain and DNA Base† [J]. Chem. J. Chinese Universities, 2017, 38(1): 56. |
| [3] | SUN Xiaoli, HUO Ruiping, BU Yuxiang, LI Jilai. Benchmark Studies of Density Functional Theory on the Hydrogen Adsorption† [J]. Chem. J. Chinese Universities, 2015, 36(8): 1570. |
| [4] | WANG Xiaowen, LI Shuang, JIANG Xiaonan, WANG Changsheng. Site-preference of Quercetin Hydrogen Bonding to Adenine† [J]. Chem. J. Chinese Universities, 2015, 36(5): 932. |
| [5] | LIU Cui, ZHANG Qianhui, GONG Lidong, LU Linan, YANG Zhongzhi. Theoretical Studies on the Effect of Fapy-G on Base Pair Hydrogen Bond Complexes† [J]. Chem. J. Chinese Universities, 2014, 35(12): 2645. |
| [6] | LIU Peng, LI Shushi, WANG Changsheng. Effects of Substituents on the Binding Energy in Hydrogen-bonded Complexes Containing Adenine and Thymine† [J]. Chem. J. Chinese Universities, 2014, 35(1): 154. |
| [7] | ZHANG Ji, LI Hai-Bin, WU Yong, GENG Yun, DUAN Yu-Ai, LIAO Yi*, SU Zhong-Min*. TD-DFT Studies on Phenothiazine-based Dyes with Different Donor in Dye-sensitized Solar Cells [J]. Chem. J. Chinese Universities, 2011, 32(6): 1343. |
| [8] | HUO Hong-Jie, ZHAO Dong-Xia*, YANG Zhong-Zhi* . Theoretical Study on the Interaction between Bases and NMA by Ab initio and ABEEMσπ Methods [J]. Chem. J. Chinese Universities, 2011, 32(12): 2877. |
| [9] | ZHANG Wen-Long, CHEN Shu-Ling, YANG Zhong-Zhi*. Calculation of Complexes of the Recombinant Kringle 1 Domain of Human Plasminogen and Its Ligands by ABEEMσπ/MM Method [J]. Chem. J. Chinese Universities, 2010, 31(8): 1630. |
| [10] | NI Zhe-Ming, YAO Ping, LIU Xiao-ing, WANG Qiao-iao, XU Qian. Theory Study on the Distorted Structure and Stability of Copper\|Zinc\|Aluminum Layered Double Hydroxides [J]. Chem. J. Chinese Universities, 2010, 31(12): 2438. |
| [11] | LÜ Yan-Yan, TAN Hong-Wei*, CHEN Guang-Ju, LIU Ruo-Zhuang*. Theoretical Study on Ions Selectivity of Calmodulin [J]. Chem. J. Chinese Universities, 2008, 29(12): 2345. |
| [12] | JIN Lian-Ji, ZHANG Min, SU Zhong-Min*, SHI Li-Li, ZHAO Liang. Theoretical Study on Hydrocarbon Molecule(Acetylene, Ethylene, Ethane) Doped Armchair Single-Walled Carbon Nanotube [J]. Chem. J. Chinese Universities, 2007, 28(4): 755. |
| [13] | CHEN Zhao-Xing, LI Qin-Yu, XU Xuan, ZENG He-Ping. Quantum Chemistry Studies on Halogen-benzylidene-quinolin-8-ol Alumium Complex [J]. Chem. J. Chinese Universities, 2007, 28(2): 338. |
| [14] | LIU Wei, GONG Jie, YANG Jing-Hai, ZONG Zhan-Guo, CHEN Gang . Synthesis and Structure Evolution of LiNi1-xAlxO2(x=0—0.6) [J]. Chem. J. Chinese Universities, 2005, 26(8): 1484. |
| [15] | CHEN Lan-Hui, JIN Lian-Ji, SU Zhong-Min, QIU Yong-Qing, WANG Yong, LIU Shu-Ying. ESI-MSn Behavior and Quantum Chemistry Calculation of Stability of Fragment Ions of Diester-diterpenoid Alkaloids(DDA) [J]. Chem. J. Chinese Universities, 2005, 26(12): 2340. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||