

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (5): 932.doi: 10.7503/cjcu20141136
• Physical Chemistry • Previous Articles Next Articles
WANG Xiaowen, LI Shuang, JIANG Xiaonan, WANG Changsheng*( )
)
Received:2014-12-29
Online:2015-05-10
Published:2015-04-14
Contact:
WANG Changsheng
E-mail:chwangcs@lnnu.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Xiaowen, LI Shuang, JIANG Xiaonan, WANG Changsheng. Site-preference of Quercetin Hydrogen Bonding to Adenine†[J]. Chem. J. Chinese Universities, 2015, 36(5): 932.
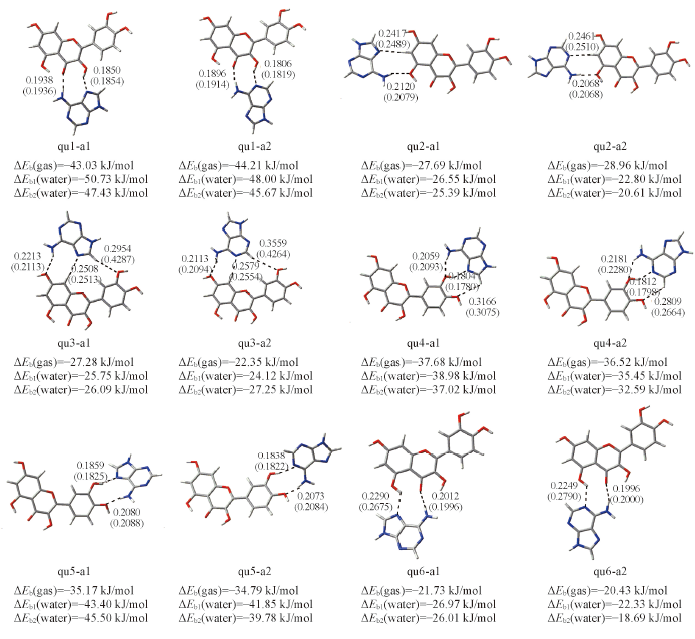
Fig.2 Optimal structures of twelve hydrogen-bonded quercetin-adenine complexesThe hydrogen bond distances in gas phase and in water solvent are given in the corresponding position and in parentheses, respectively. ΔEb(gas) is the binding energy in gas phase. ΔEb1(water) and ΔEb2(water) are the binding energies in water solvent using the optimal structure in gas phase and the optimal structure in water solvent, respectively. The hydrogen bond distance units are in nm.
| Complex | Hydrogen bond | R(Y…H)/nm | ΔE(2)/(kJ·mol-1) | ∑ΔE(2)/(kJ·mol-1) | ρc/a.u. | ∑ρc/a.u. |
|---|---|---|---|---|---|---|
| qu1-a1 | N—H…O═C | 0.1938 | 41.21 | 135.22 | 0.023 | 0.059 |
| O—H…N | 0.1850 | 94.01 | 0.036 | |||
| qu1-a2 | N—H…O═C | 0.1896 | 52.47 | 164.14 | 0.025 | 0.067 |
| O—H…N | 0.1806 | 111.67 | 0.041 | |||
| qu2-a1 | N—H…O | 0.2120 | 27.32 | 45.73 | 0.016 | 0.028 |
| C—H…N | 0.2417 | 18.41 | 0.012 | |||
| qu2-a2 | N—H…O | 0.2068 | 33.39 | 50.42 | 0.017 | 0.029 |
| C—H…N | 0.2461 | 17.03 | 0.011 | |||
| qu3-a1 | N—H…O | 0.2213 | 19.08 | 34.87 | 0.013 | 0.026 |
| C—H…O | 0.2954 | 1.23 | 0.003 | |||
| C—H…N | 0.2508 | 14.56 | 0.010 | |||
| qu3-a2 | N—H…O | 0.2113 | 28.28 | 40.20 | 0.016 | 0.025 |
| C—H…O | 0.3559 | 0.00 | 0.000 | |||
| C—H…N | 0.2579 | 11.92 | 0.008 | |||
| qu4-a1 | N—H…O | 0.2059 | 29.87 | 139.45 | 0.018 | 0.059 |
| C—H…O | 0.3166 | 0.00 | 0.000 | |||
| O—H…N | 0.1804 | 109.58 | 0.041 | |||
| qu4-a2 | N—H…O | 0.2181 | 15.56 | 125.26 | 0.014 | 0.060 |
| C—H…O | 0.2809 | 1.17 | 0.005 | |||
| O—H…N | 0.1812 | 108.53 | 0.041 | |||
| qu5-a1 | O—H…N | 0.1859 | 89.12 | 122.84 | 0.035 | 0.053 |
| N—H…O | 0.2080 | 33.72 | 0.018 | |||
| qu5-a2 | O—H…N | 0.1838 | 98.53 | 131.58 | 0.038 | 0.057 |
| N—H…O | 0.2073 | 33.05 | 0.018 | |||
| qu6-a1 | N—H…O═C | 0.2012 | 32.59 | 55.60 | 0.019 | 0.033 |
| O—H…N | 0.2290 | 23.01 | 0.014 | |||
| qu6-a2 | N—H…O═C | 0.1996 | 36.11 | 61.42 | 0.019 | 0.035 |
| O—H…N | 0.2249 | 25.31 | 0.016 |
Table 1 n→σ* second-order stabilization energies ΔE(2) obtained at the B3LYP/6-31G(d,p) level and the electron densities(ρc) at the hydrogen bond critical points obtained at the B3LYP/6-311+G(d,p) level
| Complex | Hydrogen bond | R(Y…H)/nm | ΔE(2)/(kJ·mol-1) | ∑ΔE(2)/(kJ·mol-1) | ρc/a.u. | ∑ρc/a.u. |
|---|---|---|---|---|---|---|
| qu1-a1 | N—H…O═C | 0.1938 | 41.21 | 135.22 | 0.023 | 0.059 |
| O—H…N | 0.1850 | 94.01 | 0.036 | |||
| qu1-a2 | N—H…O═C | 0.1896 | 52.47 | 164.14 | 0.025 | 0.067 |
| O—H…N | 0.1806 | 111.67 | 0.041 | |||
| qu2-a1 | N—H…O | 0.2120 | 27.32 | 45.73 | 0.016 | 0.028 |
| C—H…N | 0.2417 | 18.41 | 0.012 | |||
| qu2-a2 | N—H…O | 0.2068 | 33.39 | 50.42 | 0.017 | 0.029 |
| C—H…N | 0.2461 | 17.03 | 0.011 | |||
| qu3-a1 | N—H…O | 0.2213 | 19.08 | 34.87 | 0.013 | 0.026 |
| C—H…O | 0.2954 | 1.23 | 0.003 | |||
| C—H…N | 0.2508 | 14.56 | 0.010 | |||
| qu3-a2 | N—H…O | 0.2113 | 28.28 | 40.20 | 0.016 | 0.025 |
| C—H…O | 0.3559 | 0.00 | 0.000 | |||
| C—H…N | 0.2579 | 11.92 | 0.008 | |||
| qu4-a1 | N—H…O | 0.2059 | 29.87 | 139.45 | 0.018 | 0.059 |
| C—H…O | 0.3166 | 0.00 | 0.000 | |||
| O—H…N | 0.1804 | 109.58 | 0.041 | |||
| qu4-a2 | N—H…O | 0.2181 | 15.56 | 125.26 | 0.014 | 0.060 |
| C—H…O | 0.2809 | 1.17 | 0.005 | |||
| O—H…N | 0.1812 | 108.53 | 0.041 | |||
| qu5-a1 | O—H…N | 0.1859 | 89.12 | 122.84 | 0.035 | 0.053 |
| N—H…O | 0.2080 | 33.72 | 0.018 | |||
| qu5-a2 | O—H…N | 0.1838 | 98.53 | 131.58 | 0.038 | 0.057 |
| N—H…O | 0.2073 | 33.05 | 0.018 | |||
| qu6-a1 | N—H…O═C | 0.2012 | 32.59 | 55.60 | 0.019 | 0.033 |
| O—H…N | 0.2290 | 23.01 | 0.014 | |||
| qu6-a2 | N—H…O═C | 0.1996 | 36.11 | 61.42 | 0.019 | 0.035 |
| O—H…N | 0.2249 | 25.31 | 0.016 |
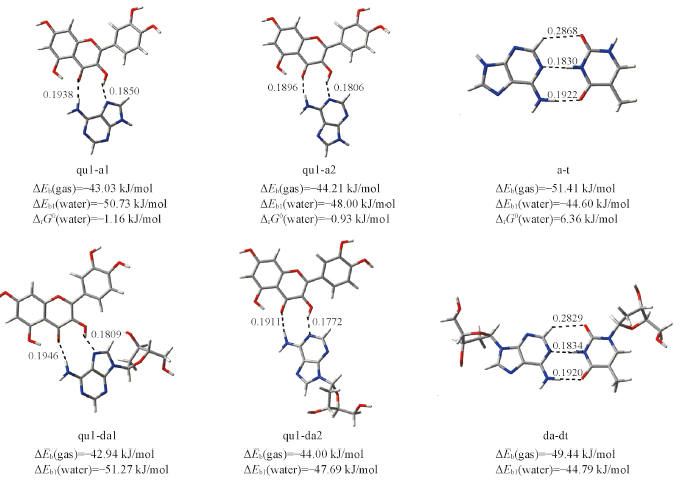
Fig.3 Optimal structures of six hydrogen-bonded complexesThe hydrogen bond distances are given in the corresponding position. ΔEb(gas) and ΔEb1(water) are the binding energies in gas phase and in water solvent using the optimal structure in gas phase. ΔrG0(water) is the standard Gibbs free energy change in water solvent using the optimal structure in water solvent. The bond distance units are in nm.
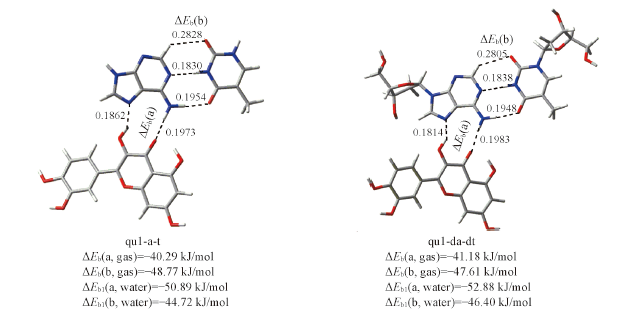
Fig.4 Optimal structures of the hydrogen-bonded quercetin-adenine-thymine and quercetin-deoxyadenosine-deoxythymidine complexesThe hydrogen bond distances are given in the corresponding position. ΔEb(gas) and ΔEb1(water) are the binding energies in gas phase and in water solvent using the optimal structure in gas phase. The bond distance units are in nm.
| [1] | Rachel E., A. , Neil, O. , Chem. Res. Toxicol., 2014, 27( 5), 787- 793 |
| [2] | Soumya S., M. , Somnath S., R. , Sayantani, C. , Sudin, B. , Subhash C., B. , J. Phys. Chem. B, 2013, 117( 47), 14655- 14665 |
| [3] | Eileen S., K. , Zhong J., C. , James A., H. , Biochemistry, 2013, 52( 9), 1559- 1567 |
| [4] | 张长胜, 来鲁华. 物理化学学报, 2012, 28( 10), 2363- 2380 |
| Zhang C., S. , Lai L., H. , Acta Phys.-Chim. Sin., 2012, 28( 10), 2363- 2380 | |
| [5] | 于楠, 刘朋, 王长生. 物理化学学报, 2013, 29( 6), 1173- 1182 |
| Yu, N. , Liu, P. , Wang C., S. , Acta Phys.-Chim. Sin., 2013, 29( 6), 1173- 1182 | |
| [6] | Adam C., K. , William A., D. , David E., G. , Neil, O. , Biochemistry, 2012, 51( 8), 1730- 1739 |
| [7] | Steven L., P. , Michae J., J. , Maria, D. , Daniel, D. , David E., G. , Neil, O. , Biochemistry, 2011, 50( 22), 5058- 5066 |
| [8] | Mohajeri, A. , Nobandegani F., F. , J. Phys. Chem. A, 2008, 112( 2), 281- 295 |
| [9] | Kawahara, S. , Uchimaru, T. , Tairi, K. , Sekine, M. , J. Phys. Chem. A, 2002, 106( 13), 3207- 3212 |
| [10] | 蓝蓉, 李浩然, 韩世钧. 化学学报, 2005, 63( 14), 1288- 1292 |
| Lan, R. , Li H., R. , Han S., J. , Acta Chim. Sinica, 2005, 63( 14), 1288- 1292 | |
| [11] | Asensio, A. , Kobko, N. , Dannenberg J., J. , J. Phys. Chem. A, 2003, 107( 33), 6441- 6443 |
| [12] | 林雪飞, 孙成科, 杨思娅, 余仕问, 姚立峰, 陈益山. 化学学报, 2011, 69( 23), 2787- 2795 |
| Lin X., F. , Sun C., K. , Yang S., Y. , Yu S., W. , Yao L., F. , Chen Y., S. , Acta Chim. Sinica, 2011, 69( 23), 2787- 2795 | |
| [13] | Li, Y. , Wang C., S. , Sci. China Ser. Chem., 2011, 54( 11), 1759- 1769 |
| [14] | Huang C., Y. , Li, Y. , Wang C., S. , Sci. China Chem., 2013, 56( 2), 238- 248 |
| [15] | Jiang X., N. , Wang C., S. , Sci. China Ser. Chem., 2010, 53( 8), 1754- 1761 |
| [16] | Zhao G., J. , Liu J., Y. , Zhou L., C. , Han K., L. , J. Phys. Chem. B, 2007, 111( 30), 8940- 8945 |
| [17] | Zhao G., J. , Han K., L. , Accounts Chem. Res., 2012, 45( 3), 404- 413 |
| [18] | Li S., S. , Huang C., Y. , Hao J., J. , Wang C., S. , J. Comput. Chem., 2014, 35( 6), 415- 506 |
| [19] | Dong, H. , Hua W., J. , Li S., H. , J. Phys. Chem. A, 2007, 111( 15), 2941- 2945 |
| [20] | Hao J., J. , Li S., S. , Jiang X., N. , Li X., L. , Wang C., S. , Theor. Chem. Acc., 2014, 133, 1516- 1527 |
| [21] | Wu Y., D. , Zhao Y., L. , J. Am. Chem. Soc., 2001, 123( 22), 5313- 5319 |
| [22] | 刘朋, 李书实, 王长生. 高等学校化学学报, 2014, 35( 1), 154- 160 |
| Liu, P. , Li S., S. , Wang C., S. , Chem. J. Chinese Universities, 2014, 35( 1), 154- 160 | |
| [23] | Li, Y. , Wang C., S. , J. Comput Chem., 2011, 32( 13), 2765- 2773 |
| [24] | Hao J., J. , Wang C., S. , RSC Adv., 2015, 5, 6452- 6461 |
| [25] | Riham I., E. , Noelia, R. , Julie T. W., W. , Wafa T., A. , Maxime, B. , Houmam, K. , Muniba, N. , Rebecca, K. , Vincenzo, A. , Fré, déric L. , Sara, B. , Gustaaf V., T. , Amany O., K. , Gehanne A. S., A. , Nahed D., M. , Khuloud T., A. , ACS Nano, 2014, 8( 2), 1384- 1401 |
| [26] | Yu C., H. , Hui T., Y. , Nan W., S. , J. Agric. Food Chem., 2012, 60( 10), 2674- 2681 |
| [27] | Lespade, L. , Bercion, S. , J. Phys. Chem. B, 2010, 114( 2), 921- 928 |
| [28] | Lekka C., E. , Ren, J. , Meng, S. , Kaxiras, E. , J. Phys. Chem. B, 2009, 113( 18), 6478- 6483 |
| [29] | Havsteen B., H. , Pharmacol. Ther., 2002, 96( 2/3), 67- 202 |
| [30] | Leopoldini, M. , Marino, T. , Russo, N. , Toscano, M. , Theor. Chem. Acc. , 2004, 111( 2/6), 210- 216 |
| [31] | Bouktaib, M. , Lebrun, S. , Atmani, A. , Rolando, C. , Tetrahedron., 2002, 58( 50), 10001- 10009 |
| [32] | Hä, mäläinen M. , Nieminen, R. , Vuorela, P. , Mediators Inflamm., 2007, 2007, 45673- 45682 |
| [33] | Bidisha, S. , Samantha M., R. , Donald E. D., J. , Kisa, H. , Randy M., W. , Denise, W. , D’, Asia G. , Cari, H. , J. Phys. Chem. B, 2015, 119( 6), 2546- 2556 |
| [34] | Omari J., B. , Sara J., C. , Neil, O. , Chem. Res. Toxicol., 2008, 21, 1253- 1260 |
| [35] | Zhang X., M. , Huang S., P. , Xu, Q. , Cancer Chemother. Pharmacol., 2004, 53( 1), 82- 88 |
| [36] | Becke A., D. , J. Chem. Phys., 1993, 98, 5648- 5652 |
| [37] | Lee, C. , Yang W., T. , Parr R., G. , Phys. Rev. B, 1988, 37, 785- 789 |
| [38] | Mϕ, ller C. , Plesset M., S. , Phys. Rev., 1934, 46, 618 |
| [39] | York D., M. , Karplus, M. , J. Phys. Chem. A, 1999, 103( 50), 11060- 11079 |
| [40] | Frisch M., J. , Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T. J. A., Montgomery J., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Keith T., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision D. 01, Gaussian, Inc., Wallingford CT, 2013. |
| [41] | Biegler K., F. , Schonbohm, J. , Bayles, D. , J. Comput. Chem., 2001, 22( 5), 545- 559 |
| [1] | LI Xiaolei, SUN Yunjiao, TANG Ying, WANG Changsheng. Rapid and Accurate Calculation of the Three⁃body Interaction Strength in the Hydrogen⁃bonded Complexes of Alcohols or Deoxyribose with Water [J]. Chem. J. Chinese Universities, 2021, 42(12): 3664. |
| [2] | LI Fei, LI Xiaoxuan, LI Yijun, HE Xiwen, CHEN Langxing, ZHANG Yukui. Preparation of Surface Oriented Magnetically Imprinted Polymers and the Selective Recognition of Quercetin [J]. Chem. J. Chinese Universities, 2021, 42(12): 3606. |
| [3] | YAN Fanyong, SUN Zhonghui, PANG Jiping, JIANG Yingxia, CHEN Yuan. Functionalized Carbon Dots of Benzothiazine Derivatives for Detection of Quercetin in Ginkgo Biloba Tea [J]. Chem. J. Chinese Universities, 2020, 41(8): 1768. |
| [4] | WANG Mengyu, CAO Simin, LI Haoyang, ZHANG Mengjie, LI Dong, ZHAO Zenan, XU Jianhua. Fluorescence Resonance Energy Transfer Between Coenzyme NADH and Tryptophan [J]. Chem. J. Chinese Universities, 2020, 41(11): 2473. |
| [5] | LI Jinxing,XING Xiaofeng,QI Zhongnan,AI Hongqi. Effects of Three New Modified Molecules on the Structural Stability of Different Aβ42 Fibers† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2230. |
| [6] | LI Lei,HUANG Cuiying,JIANG Xiaonan,GAO Xichan,WANG Changsheng. Ionic Hydrogen Bonding Between Arginine Side Chain and Nucleic Acid Bases† [J]. Chem. J. Chinese Universities, 2016, 37(8): 1460. |
| [7] | SUN Xiaoli, HUO Ruiping, BU Yuxiang, LI Jilai. Benchmark Studies of Density Functional Theory on the Hydrogen Adsorption† [J]. Chem. J. Chinese Universities, 2015, 36(8): 1570. |
| [8] | TURSON Mamat, DAWUT Gulbahar, EMIN Risalat, CHU Ganghui, JELIL Mahmutjan, TURHON Muhetar. Preparation of Quercetin Imprinted Polymer by Living Radical Polymerization and Its Application in the Composition Analysis of Zukamu Granules for Uighur Medicine† [J]. Chem. J. Chinese Universities, 2015, 36(12): 2402. |
| [9] | LIU Cui, ZHANG Qianhui, GONG Lidong, LU Linan, YANG Zhongzhi. Theoretical Studies on the Effect of Fapy-G on Base Pair Hydrogen Bond Complexes† [J]. Chem. J. Chinese Universities, 2014, 35(12): 2645. |
| [10] | LI Shushi, JIANG Xiaonan, WANG Changsheng. Three-body Effect in Hydrogen-bonded Complexes Containing Amides and Uracil† [J]. Chem. J. Chinese Universities, 2014, 35(11): 2403. |
| [11] | LIU Peng, LI Shushi, WANG Changsheng. Effects of Substituents on the Binding Energy in Hydrogen-bonded Complexes Containing Adenine and Thymine† [J]. Chem. J. Chinese Universities, 2014, 35(1): 154. |
| [12] | WANG Lei, FU Ling, JING Lin-Lin, A You-Mei, JIA Lu. Studies of Chemical Constituents from Rosa chinensis Jacq. [J]. Chem. J. Chinese Universities, 2012, 33(11): 2457. |
| [13] | CHEN Zhe, KWONG Anna Ka-Yee, YANG Zhen-Jun, ZHANG Liang-Ren, LEE Hon Cheung, ZHANG Li-He. Synthesis and Biological Evaluation of Nicotinamide Adenine Dinucleotides Analogues as Inhibitors of CD38 [J]. Chem. J. Chinese Universities, 2012, 33(06): 1226. |
| [14] | ZHANG Ji, LI Hai-Bin, WU Yong, GENG Yun, DUAN Yu-Ai, LIAO Yi*, SU Zhong-Min*. TD-DFT Studies on Phenothiazine-based Dyes with Different Donor in Dye-sensitized Solar Cells [J]. Chem. J. Chinese Universities, 2011, 32(6): 1343. |
| [15] | CHEN Jin-Peng, QI Zhong-Nan, YANG Ai-Bin, AI Hong-Qi*. Stability of the Complexes Combined by Metal Ions and Adenine Base Isomers [J]. Chem. J. Chinese Universities, 2011, 32(5): 1169. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||