

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (11): 2445.doi: 10.7503/cjcu20180168
• Organic Chemistry • Previous Articles Next Articles
DU Shanshan1, LI Yang1, GUO Lei2, LI Pengyu1, CHAI Zhilong1, WANG Tao2, QUAN Dongqin2, HE Junlin2,*( )
)
Received:2018-03-04
Online:2018-11-10
Published:2018-10-10
Contact:
HE Junlin
E-mail:hejunlin@bmi.ac.cn
Supported by:CLC Number:
TrendMD:
DU Shanshan, LI Yang, GUO Lei, LI Pengyu, CHAI Zhilong, WANG Tao, QUAN Dongqin, HE Junlin. Modification of Aptamer TBA with Extra Functional Groups and the Biological Activities†[J]. Chem. J. Chinese Universities, 2018, 39(11): 2445.
| Name | Sequences d(5'-3') | MS, m/z(calcd. ) |
|---|---|---|
| TBA | GGT TGG TGT GGT TGG | 4727.4(4726.0) |
| TBA-T3-1 | GG1 TGG TGT GGT TGG | 4813.8(4812.1) |
| TBA-T4-1 | GGT1GG TGT GGT TGG | 4814.0(4812.1) |
| TBA-T12-1 | GGT TGG TGT GG1 TGG | 4814.3(4812.1) |
| TBA-T13-1 | GGT TGG TGT GGT1GG | 4815.4(4812.1) |
| TBA-T3-T12-1 | GG1 TGG TGT GG1 TGG | 4899.1(4898.2) |
| TBA-T4-T13-1 | GGT1GG TGT GGT 1GG | 4898.9(4898.2) |
Table 1 TBA and its analogues modified with the monomer 1 and the mass spectra data
| Name | Sequences d(5'-3') | MS, m/z(calcd. ) |
|---|---|---|
| TBA | GGT TGG TGT GGT TGG | 4727.4(4726.0) |
| TBA-T3-1 | GG1 TGG TGT GGT TGG | 4813.8(4812.1) |
| TBA-T4-1 | GGT1GG TGT GGT TGG | 4814.0(4812.1) |
| TBA-T12-1 | GGT TGG TGT GG1 TGG | 4814.3(4812.1) |
| TBA-T13-1 | GGT TGG TGT GGT1GG | 4815.4(4812.1) |
| TBA-T3-T12-1 | GG1 TGG TGT GG1 TGG | 4899.1(4898.2) |
| TBA-T4-T13-1 | GGT1GG TGT GGT 1GG | 4898.9(4898.2) |
| Name | Tm/℃ | Name | Tm/℃ |
|---|---|---|---|
| TBA | 35.0 | TBA-T13-1 | 30.6 |
| TBA-T3-1 | 36.1 | TBA-T3-T12-1 | 33.7 |
| TBA-T4-1 | 30.5 | TBA-T4-T13-1 | 27.4 |
| TBA-T12-1 | 34.6 |
Table 2 Thermal stability of TBA and its analogues*
| Name | Tm/℃ | Name | Tm/℃ |
|---|---|---|---|
| TBA | 35.0 | TBA-T13-1 | 30.6 |
| TBA-T3-1 | 36.1 | TBA-T3-T12-1 | 33.7 |
| TBA-T4-1 | 30.5 | TBA-T4-T13-1 | 27.4 |
| TBA-T12-1 | 34.6 |
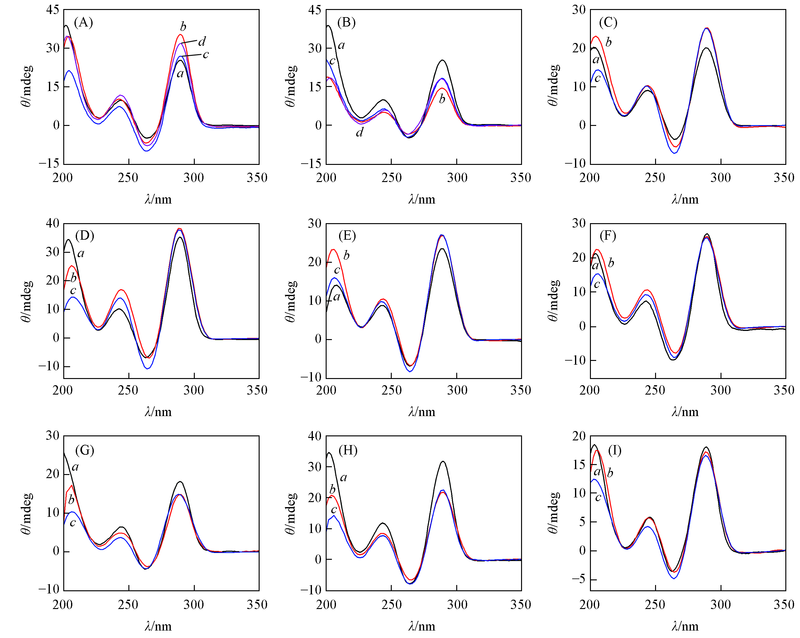
Fig. 1 CD spectra of TBA and its analogue(A), (B) in PBS(138 mmol/L NaCl+27 mmol/L KCl+10 mmol/L Na2HPO4+1.76 mmol/L KH2PO4, pH=7.4); (C)—(I) CD spectra of TBA and its analogue in the presence of thrombin(6 U, with incubation time of 5 min(labeled with a) and overnight(labeled with b) in PBS. (A) a. TBA; b. TBA-T3-1; c. TBA-T12-1; d. TBA-T3-T12-1; (B) a. TBA; b. TBA-T4-1; c. TBA-T13-1; d. TBA-T4-T13-1; (C) a. TBA; b. TBA-a; c. TBA-b; (D) a. TBA-T3-1; b. TBA-T3-1-a; c. TBA-T3-1-b; (E) a. TBA-T4-1; b. TBA-T4-1-a; c. TBA-T4-1-b; (F) a. TBA-T12-1; b. TBA-T12-1-a; c. TBA-T12-1-b; (G) a. TBA-T13-1; b. TBA-T13-1-a; c. TBA-T13-1-b; (H) a. TBA-T3-T12-1; b. TBA-T3-T12-1-a; c. TBA-T3-T12-1-b; (I) a. TBA-T4-T13-1; b. TBA-T4-T13-1-a; c. TBA-T4-T13-1-b.
| Name | Clotting time/s | Anticoagulant effect/s | Name | Clotting time/s | Anticoagulant effect/s |
|---|---|---|---|---|---|
| Control | 20.1±0.2 | | TBA-T12-1 | 31.2±0.6 | 11.1 |
| TBA | 28.4±0.1 | 8.3 | TBA-T13-1 | 24.4±0.1 | 4.3 |
| TBA-T3-1 | 30.6±0.4 | 10.5 | TBA-T3-T12-1 | 27.6±0.6 | 7.5 |
| TBA-T4-1 | 23.6±0.5 | 3.5 | TBA-T4-T13-1 | 24.3±0.2 | 4.2 |
Table 3 Anti-anticoagulant effect of TBA and its analogues
| Name | Clotting time/s | Anticoagulant effect/s | Name | Clotting time/s | Anticoagulant effect/s |
|---|---|---|---|---|---|
| Control | 20.1±0.2 | | TBA-T12-1 | 31.2±0.6 | 11.1 |
| TBA | 28.4±0.1 | 8.3 | TBA-T13-1 | 24.4±0.1 | 4.3 |
| TBA-T3-1 | 30.6±0.4 | 10.5 | TBA-T3-T12-1 | 27.6±0.6 | 7.5 |
| TBA-T4-1 | 23.6±0.5 | 3.5 | TBA-T4-T13-1 | 24.3±0.2 | 4.2 |
| [1] | Ellington A. D., Szostak J. W., Nature, 1990, 346, 818—822 |
| [2] | Tuerk C., Gold L., Science, 1990, 249, 505—510 |
| [3] | Gao L., Wang Q., Yang X. H., Wang K. M., Deng P., Zhang H., Li Z. P., Chem. J. Chinese Universities, 2017, 38(1), 187—192 |
| (高蕾, 王青, 羊小海, 王柯敏, 邓鹏, 张华, 李志平. 高等学校化学学报, 2017, 38(1), 187—192) | |
| [4] | Neves M. A. D., Blaszykowski C., Thompson M., Anal. Chem., 2016, 88, 3098—3106 |
| [5] | Yu J., Zhang L., Xu X., Liu S., Anal. Chem., 2014, 86, 10741—10748 |
| [6] | Benedetto G., Hamp T. J., Wesselman P. J., Richardson C., Nucleic Acid Ther., 2015, 25, 162—172 |
| [7] | Liu Z. C., Zhang Y. F., Xie Y., Sun Y., Bi K. W., Cui Z., Zhao L. J., Fan W. F., Chem. Res. Chinese Universities, 2017, 33(5), 714—720 |
| [8] | Trausch J. J., Shank-Retzlaff M., Verch T., Anal. Chem., 2017, 89, 3554—3561 |
| [9] | Yu M., Wang H., Fu F., Li L., Li J., Li G., Song Y., Swihart M. T., Song E., Anal. Chem., 2017, 89, 4085—4090 |
| [10] | Famulok M., Mayer G., Acc. Chem. Res., 2011, 44, 1349—1358 |
| [11] | Eissa S., Zourob M., Anal. Chem., 2017, 89, 3138—3145 |
| [12] | Han Y., Diao D., Lu Z., Li X., Guo Q., Huo Y., Xu Q., Li Y., Cao S., Wang J., Wang Y., Zhao J., Li Z., He M., Luo Z., Lou X., Anal. Chem., 2017, 89, 5270—5277 |
| [13] | Hassan E. M., Willmore W. G., DeRosa M. C., Nucleic Acid Ther., 2016, 26(6), 335—347 |
| [14] | Maier K. E., Levy M., Mol. Ther. Methods Clin. Develop., 2016, 5, 16014 |
| [15] | Nimjee S. M., Rusconi C. P., Sullenger B. A., Annu. Rev. Med., 2005, 56, 555—583 |
| [16] | Mayer G., Rohrbach F., Pötzsch B., Müller J., Hämostaseologie, 2011, 31, 258—263 |
| [17] | Bock L. C., Griffin L. C., Latham J. A., Vermaas E. H., Toole J. J., Nature, 1992, 355, 564—566 |
| [18] | Griffin L. C., Tidmarsh G. F., Bock L. C., Toole J. J., Leung L. L. K., Blood, 1993, 81(12), 3271—3276 |
| [19] | Müller J., Freitag D., Mayer G., Pötzsch B., J. Thromb. Haemost., 2008, 6, 2105—2112 |
| [20] | Bai Y., Li Y., Zhang D., Wang H., Zhao Q., Anal. Chem., 2017, 89, 9467—9473 |
| [21] | Aviñó A., Fàbrega C., Tintoré M., Eritja R., Curr. Pharm. Des., 2012, 18, 2036—2047 |
| [22] | Nagatoishi S., Isono N., Tsumoto K., Sugimoto N., Biochiemie, 2011, 93, 1231—1238 |
| [23] | Zaitseva M., Kaluzhny D., Shchyolkina A., Borisova O., Smirnov I., Pozmogova G., Biophys. J., 2010, 146, 1—6 |
| [24] | Pagano B., Martino L., Randazzo A., Giancola C., Biophys. J., 2008, 94, 562—569 |
| [25] | Padmanabhan K., Padmanabhan K. P., Ferrara J. D., Sadler J. E., Tulinsky A., J. Biol. Chem., 1993, 268, 17651—17654 |
| [26] | Krauss I. R., Merlino A., Randazzo A., Novellino E., Mazzarella L., Sica F., Nucleic Acids Res., 2012, 40, 8119—8128 |
| [27] | Macaya R. F., Schultze P., Smith F. W., Roe J. A., Feigon J., Proc. Natl. Acad. Sci. USA, 1993, 90, 3745—3749 |
| [28] | Kelly J. A., Feigon J., Yeates T. O., J. Mol. Biol., 1996, 256, 417—422 |
| [29] | Cai B., Yang X., Sun L., Fan X., Li X., Jin H., Wu Y., Guan Z., Zhang L., Zhang L., Yang Z., Org. Biomol. Chem., 2014, 12, 8866—8876 |
| [30] | Borbone N., Bucci M., Oliviero G., Morelli E., Amato J., D'Atri V., D'Errico S., Vellecco V., Cirino G., Piccialli G., Fattorusso C., Varra M., Mayol L., Persico M., Scuotto M., J. Med. Chem., 2012, 55, 10716—10728 |
| [31] | Hernandez F. J., Kalra N., Wengel J., Vester B., Bioorg. Med. Chem. Lett., 2009, 19, 6585—6587 |
| [32] | Virno A., Randazzo A., Giancola C., Bucci M., Cirino G., Mayol L., Bioorg. Med. Chem., 2007, 15, 5710—5718 |
| [33] | Bonifacio L., Church F., Jarstfer M., Int. J. Mol. Sci., 2008, 9, 422—433 |
| [34] | Virgilio A., Petraccone L., Scuotto M., Vellecco V., Bucci M., Mayol L., Varra M., Esposito V., Galeone A., ChemBioChem, 2014, 15, 2427—2434 |
| [35] | Kong D., Yeung W., Hili R., J. Am. Chem. Soc., 2017, 139, 13977—13980 |
| [36] | Rohloff J. C., Gelinas A. D., Jarvis T. C., Ochsner U. A., Schneider D. J., Gold L., Janjic N., Mol. Ther. Nucleic Acids, 2014, 3, e201 |
| [37] | Li P., Du S., Li Y., He J., Molecules, 2017, 22, 1011 |
| [38] | Damha M. J., Giannaris P. A., Zabarylo S. V., Nucleic Acids Res., 1990, 18, 3813—3821 |
| [39] | Nagatoishi S., Tanaka Y., Tsumoto K., Biochem. Biophys. Res. Commun., 2007, 352, 812—817 |
| [1] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [2] | ZHANG Jun, WANG Bin, PAN Li, MA Zhe, LI Yuesheng. Synthesis and Properties of Imidazolium-based Polyethylene Ionomer [J]. Chem. J. Chinese Universities, 2020, 41(9): 2070. |
| [3] | RAN Shiya,SHEN Haifeng,LI Xiaonan,WANG Zilu,GUO Zhenghong,FANG Zhengping. Effect and Mechanism of Rare Earth Trifluoromethanesulfonate on the Thermal Stability of Polypropylene† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1333. |
| [4] | FANG Xijie,LIU Ruiyun,LIN Sen,SHI Lei,WANG Runwei,LI Yi,LI Junying. Synthesis of STW-zeotype Germanosilicate via Steam-assisted Crystallization† [J]. Chem. J. Chinese Universities, 2019, 40(5): 867. |
| [5] | YIN Mengxin,LIU Dongsheng,ZHAO Dongyue,DING Tong,TIAN Ye,LI Xingang. Effect of Copper Doping on Lean NOx Trap Performance of Pt/Ba/CuxMg1-xAl2O4 Catalysts at High Temperatures † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2170. |
| [6] | LIU Yi, XU Xiaozhou, MO Song, ZHAI Lei, HE Minhui, FAN Lin. Thermal Stability of Polyimide Resins Containing Siloxane Structure and Their High Temperature Structural Evolution [J]. Chem. J. Chinese Universities, 2019, 40(1): 187. |
| [7] | MENG Jiafeng, NI Xufeng, ZHENG Hao, SHEN Zhiquan. Copolymerization of Norbornene and 1-Octene Catalyzed by Bis(phenoxy-imine) Titanium Complex† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1853. |
| [8] | LI Yang, LI Zhiwen, ZHU Junfei, LIU Shihui, HE Junlin. Construction of Pyrenyl Pairs in dsDNA: Fluorescent Properties of Multiple Pyrenyl-attached dsDNAs Through 7-Substituted 8-Aza-7-deaza-2'-deoxyadenosine Analogues† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2206. |
| [9] | HE Xiaoqin, HE Junlin, XU Hua, GUO Lei, XIE Jianwei. Active Conformation of DNA Aptamer Against Recombinant Human Erythropoietin-α† [J]. Chem. J. Chinese Universities, 2018, 39(1): 48. |
| [10] | LI Caixin, LIANG Xiaorong, GU Ju. Preparation and Characterization of Bagasse Nanocellulose† [J]. Chem. J. Chinese Universities, 2017, 38(7): 1286. |
| [11] | LIU Yaoyao, DING Tong, ZHAO Dongyue, GAO Zhongnan, GUO Lihong, TIAN Ye, LI Xingang. Effect of Potassium Loading on the NOx Storage and Reduction Performance of the CuO/K2CO3/MgAl2O4 Catalyst at High Temperature† [J]. Chem. J. Chinese Universities, 2017, 38(11): 2006. |
| [12] | LI Wei, WU Suhua, Ren Xinxin. Effect of Co-stabilizers on the Properties of Ba/Zn System [J]. Chem. J. Chinese Universities, 2017, 38(11): 2089. |
| [13] | LIU Yaohua, LIN Yu, ZHANG Dongge, CHEN Chunlei, WU Guozhang, ZHANG Yan, LUAN Weiling. Fabrication of Natural Rubber/Chemically Reduced Graphene Oxide Nanocomposites and Nuclear Radiation Resistant Behavior† [J]. Chem. J. Chinese Universities, 2016, 37(7): 1402. |
| [14] | QIAN Liwei, LI Ji, SONG Wenqi, HU Xiaoling, GUAN Ping. Utilizing Macromolecular Chain as Functional Monomer and Crosslinker to Imprint BSA with Preserving the Structural Integrity of Template† [J]. Chem. J. Chinese Universities, 2016, 37(11): 2092. |
| [15] | XU Lanlan, ZHAO Qiqi, YU He, WANG Jingchen, WANG Huijun, YANG Qin, ZHU Huajie, LI Yan. Absolute Configuration Determination of One New Compound Trichoderol A from Trichoderma sp. Fungus† [J]. Chem. J. Chinese Universities, 2016, 37(11): 1972. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||