

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (9): 1578.doi: 10.7503/cjcu20170117
• Organic Chemistry • Previous Articles Next Articles
SUN Liwei, LUO Jingyi, TANG Shaokun*( )
)
Received:2017-02-25
Online:2017-09-10
Published:2017-06-12
Contact:
TANG Shaokun
E-mail:shktang@tju.edu.cn
Supported by:CLC Number:
TrendMD:
SUN Liwei, LUO Jingyi, TANG Shaokun. Synthesis of Dual-functionalized Ionic Liquid and Application in CO2 Absorption†[J]. Chem. J. Chinese Universities, 2017, 38(9): 1578.
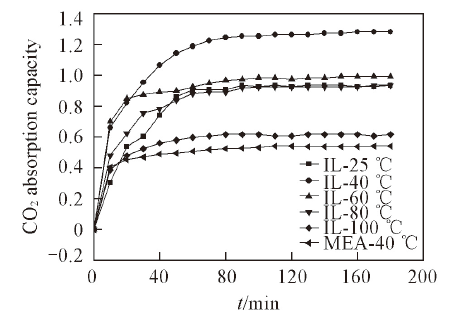
Fig.3 CO2 absorption capacity of [C3O1mim]·[Gly] and MEA vs. time at different temperaturesCO2 absorption capacity means the absorbed CO2 mol amount per mol IL.
| [1] | Dai Z. D., Noble R. D., Gin D. L., Zhang X. P., Deng L. Y., J. Membrane Sci., 2016, 497, 1—20 |
| [2] | Li Y. N., Ma R., He L. N., Diao Z. F., Catal. Sci. Technol., 2014, 4(6), 1498—1512 |
| [3] | Zhu Q. G., Sun X. F., Kang X. C., Ma J., Qian Q. L., Han B. X., Acta Phys. Chim. Sin., 2016, 32(1), 261—266 |
| (朱庆宫, 孙晓甫, 康欣晨, 马珺, 钱庆利, 韩布兴.物理化学学报,2016, 32(1), 261—266) | |
| [4] | Razali N. A., Lee K. T., Bhatia S., Mohamed A. R., Renew. Sust. Energy Rev., 2012, 16(7), 4951—4964 |
| [5] | Lan D. H., Fan N., Wang Y., Gao X., Zhang P., Chen L., Au C. T., Yin S. F., Chinese J. Catal., 2016, 37(6), 826—845 |
| (兰东辉, 樊娜, 王莹, 高显, 张平, 陈浪, 区泽堂, 尹双凤.催化学报,2016, 37(6), 826—845) | |
| [6] | Mondal M. K., Balsora H. K., Varshney P., Energy,2012, 46(1), 431—441 |
| [7] | Kenarsari S. D., Yang D. L., Jiang G. D., Zhang S. J., Wang J. J., Russell A. G., Wei Q., Fan M. H., RSC Adv., 2013, 3(45), 22739—22773 |
| [8] | Rochelle G. T., Science, 2009, 325(5948), 1652—1654 |
| [9] | Gabrielson J., Svendsen H. F., Michelsen M. L., Stenby E. H., Kontogeorgis G. M., Chem. Eng. Sci., 2007, 62(9), 2397—2413 |
| [10] | Liu Y., The Application of Ionic Liquids in Catalysis Process, Chemical Industry Press, Beijing, 2008, 1—5 |
| (刘鹰. 离子液体在催化过程中的应用. 北京: 化学工业出版社, 2008, 1—5) | |
| [11] | Wang B., Liu C. J., Wang J. D., Lei Z. K., Hu D. L., Chem. J. Chinese Universities, 2012, 33(1), 76—81 |
| (王斌, 刘晨江, 王吉德, 雷振凯, 胡东林.高等学校化学学报,2012, 33(1), 76—81) | |
| [12] | Wikes J. S., Green Chem., 2002, 4(2), 73—80 |
| [13] | Rogers R. D., Seddon K. R., Science,2003, 302(5646), 792—793 |
| [14] | Liu T. C., Tang S. K., Ji X. W., Han P., Chem. J. Chinese Universities, 2016, 37(5), 817—821 |
| (刘团春, 唐韶坤, 纪晓伟, 韩培.高等学校化学学报,2016, 37(5), 817—821) | |
| [15] | Zhang M., Tu X., Wang J., Fang T., Wang Y., Xu X., Zhang M., Chen Y., Chem. Res. Chinese Universities, 2016, 32(4), 530—533 |
| [16] | Blanchard L. A., Hancu D., Beckman E. J., Brennecke J. F., Nature,1999, 399(6731), 28—29 |
| [17] | Anthony J. L., Maginn E. J., Brennecke J. F., J. Phys. Chem. B, 2002, 106(29), 7315—7320 |
| [18] | Fredlake C. P., Crosthwaite J. M., Hert D. G., Aki S. N., Brennecke J. F., J. Chem. Eng. Data, 2004, 49(4), 954—964 |
| [19] | Anthony J. L., Anderson J. L., Maginn E. J., Brennecke J. F., J. Phys. Chem. B, 2005, 109(13), 6366—6374 |
| [20] | Bates E. D., Mayton R. D., Ntai I., Davis J. H., J. Am. Chem. Soc., 2002, 124(6), 926—927 |
| [21] | Zhang H., Zhang H. M., Wang L. J., Shen J. Y., Chem. J. Chinese Universities, 2016, 37(9), 1660—1668 |
| (张慧, 张红梅, 王连军, 沈锦优.高等学校化学学报,2016, 37(9), 1660—1668) | |
| [22] | Sistla Y. S., Khanna A., Chem. Eng. J., 2015, 273, 268—276 |
| [23] | Jiang Y. Y., Wang G. N., Zhou Z., Wu Y. T., Geng J., Zhang Z. B., Chem. Commun., 2008, 4, 505—507 |
| [24] | Gurkan B. E., Fuente J. C., Mindrup E. M., Ficke L. E., Goodrich B. F., Price E. A., Schneider W. F., Brennecke J. F., J. Am. Chem. Soc., 2010, 132(7), 2116—2117 |
| [25] | Kanakubo M., Makino T., Taniguchi T., Nokami T., Itoh T., ACS Sustain. Chem. Eng., 2016, 4(2), 525—535 |
| [26] | Zhao Y., Design and Synthesis of Ether Functionalized Ionic Liquids Immobilised on Siliga Gel and Their Desulfurization and Performance, Tianjin University, Tianjin, 2015 |
| (赵莹. 固载醚基功能化离子液体的设计合成及其脱硫性能研究, 天津: 天津大学, 2015) | |
| [27] | Wang C. M., Luo H. M., Li H. R., Zhu X., Yu B., Dai S., Chem. Eur. J., 2012, 18(7), 2153—2160 |
| [28] | Zhang X., Huang K., Xia S., Chen Y., Wu Y., Hu X., Chem. Eng. J., 2015, 274, 30—38 |
| [29] | Palomar J., Gonzalez-Miquel M., Polo A., Rodriguez F., Ind. Eng. Chem. Res., 2011, 50(6), 3452—3463 |
| [30] | Yu H., Wu Y. T., Jiang Y. Y., Zhou Z., Zhang Z. B., New J. Chem., 2009, 33(12), 2385—2390 |
| [31] | Sharma P., Park S. D., Park K. T., Jeong S. K., Nam S. C., Baek. I. H., Bull. Korean Chem. Soc., 2012, 33(7), 2325—2332 |
| [32] | Wu Y. L., Jiao Z., Wang G. N., Wu Y. T., Zhang Z. B., Fine Chem., 2007, 24(4), 324—327 |
| (吴永良, 焦真, 王冠楠, 吴有庭, 张志炳.精细化工,2007, 24(4), 324—327) | |
| [33] | Zhang J., Zhang S., Dong K., Zhang Y., Shen Y., Lv X., Chem. Eur. J., 2006, 12(15), 4021—4026 |
| [34] | Lv L., Jiang X., Jia L., Ai T., Wu H., Chem. Res. Chinese Universities, 2017, 33(1), 112—118 |
| [35] | Song G., Zhu X., Chen R., Liao Q., Ding Y., Chen L., Chem. Eng. J., 2016, 283, 175—183 |
| [1] | WU Yu, LI Xuan, YANG Hengpan, HE Chuanxin. Construction of Cobalt Single Atoms via Double-confinement Strategy for High-performance Electrocatalytic Reduction of Carbon Dioxide [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220343. |
| [2] | REN Shijie, QIAO Sicong, LIU Chongjing, ZHANG Wenhua, SONG Li. Synchrotron Radiation X-Ray Absorption Spectroscopy Research Progress on Platinum Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220466. |
| [3] | WANG Sicong, PANG Beibei, LIU Xiaokang, DING Tao, YAO Tao. Application of XAFS Technique in Single-atom Electrocatalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220487. |
| [4] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [5] | CUI Wei, ZHAO Deyin, BAI Wenxuan, ZHANG Xiaodong, YU Jiang. CO2 Absorption in Composite of Aprotic Solvent and Iron-based Ionic Liquid [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220120. |
| [6] | GUO Zhiqiang, YANG Boru, XI Chanjuan. Recent Advances in Reductive Functionalization of Carbon Dioxide with Borohydride Reagents [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220199. |
| [7] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [8] | HUANG Xiaoshun, MA Haiying, LIU Shujuan, WANG Bin, WANG Hongli, QIAN Bo, CUI Xinjiang, SHI Feng. Recent Advances on Indirect Conversion of Carbon Dioxide to Chemicals [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220222. |
| [9] | SONG Dewen, WANG Mingwang, WANG Yani, JIAO Zhenmei, NING Hui, WU Mingbo. Progress of CO2 Electroreduction to Oxalic Acid [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220248. |
| [10] | ZHAO Runyao, JI Guipeng, LIU Zhimin. Efficient Electrocatalytic CO2 Reduction over Pyrrole Nitrogen-coordinated Single-atom Copper Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220272. |
| [11] | QIU Liqi, YAO Xiangyang, HE Liangnian. Visible-light-driven Selective Reduction of Carbon Dioxide Catalyzed by Earth-abundant Metalloporphyrin Complexes [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220064. |
| [12] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [13] | ZHANG Zhen, DENG Yu, ZHANG Qinfang, YU Dagang. Visible Light-driven Carboxylation with CO2 [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220255. |
| [14] | WANG Lijun, LI Xin, HONG Song, ZHAN Xinyu, WANG Di, HAO Leiduan, SUN Zhenyu. Efficient Electrocatalytic CO2 Reduction to CO by Tuning CdO-Carbon Black Interface [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220317. |
| [15] | JI Shuangqi, JIN Zhao, GUAN Wenna, PAN Xiangyu, GUAN Tong. Preparation and Chromatographic Performance of Mixed-mode Silica Stationary Phase Modified by Double Cationic Ionic Liquid and Octadecyl Group [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220008. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||