

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (5): 973.doi: 10.7503/cjcu20180811
• Physical Chemistry • Previous Articles Next Articles
LIU Shufeng, QIU Haiyan, JIANG Tao, ZHANG Yehua, ZHAO Yun, CHENG Hanwen, CHEN Yong*( )
)
Received:2018-12-03
Online:2019-04-17
Published:2019-04-17
Contact:
CHEN Yong
E-mail:yongchen@sit.edu.cn
Supported by:CLC Number:
TrendMD:
LIU Shufeng,QIU Haiyan,JIANG Tao,ZHANG Yehua,ZHAO Yun,CHENG Hanwen,CHEN Yong. Ion Transfer Behavior of Protonated Phenazopyridine at the Liquid/Liquid Interface Modified by Functionalized Hybrid Mesoporous Silica Membrane†[J]. Chem. J. Chinese Universities, 2019, 40(5): 973.
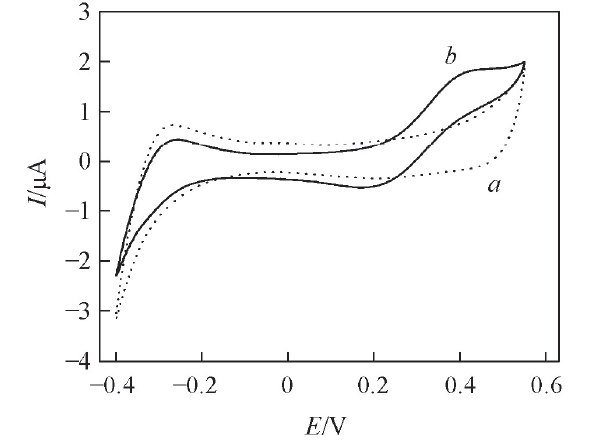
Fig.2 CVs obtained at the W/DCH interface modified by hybrid mesoporous membrane in the absence(a) and presence(b) of 0.3 mmol/L phenazopyridine hydrochloride at a scan rate of 20 mV/spH=4.0.
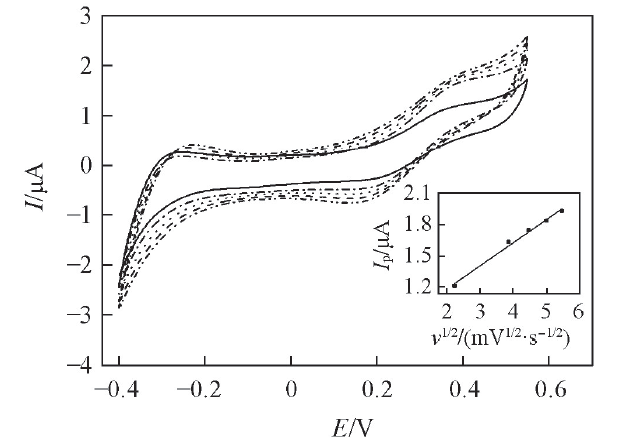
Fig.3 CVs obtained at the W/DCH interface modified by hybrid mesoporous membrane in the presence of 0.18 mmol/L phenazopyridine hydrochloride at different scan ratesFrom inner CVs to outer CVs, the scan rates are 5, 15, 20, 25 and 30 mV/s. Inset: the corresponding plot of peak current vs. the square root of scan rate.
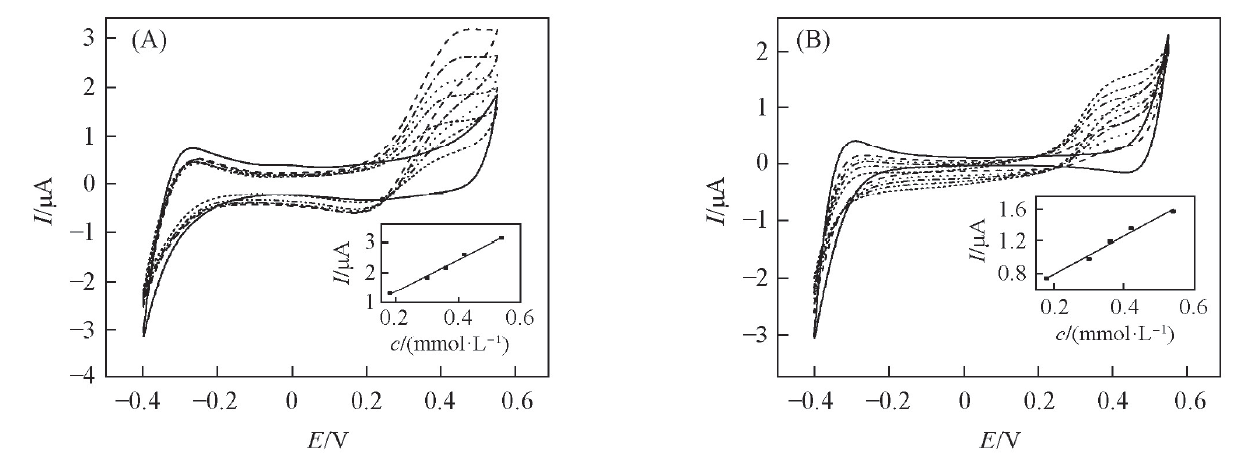
Fig.4 CVs of the W/DCH interface modified by hybrid mesoporous membrane(A) and bare PET membrane(B) at increasing concentrations of phenazopyridine hydrochlorideFrom inner CVs to outer CVs, the concentrations of phenazopyridine hydrochloride are 0, 0.18, 0.3, 0.36, 0.42 and 0.54 mmol/L. Inset: the corresponding plots of peak current vs. the concentration of phenazopyridine hydrochloride in aqueous solution.
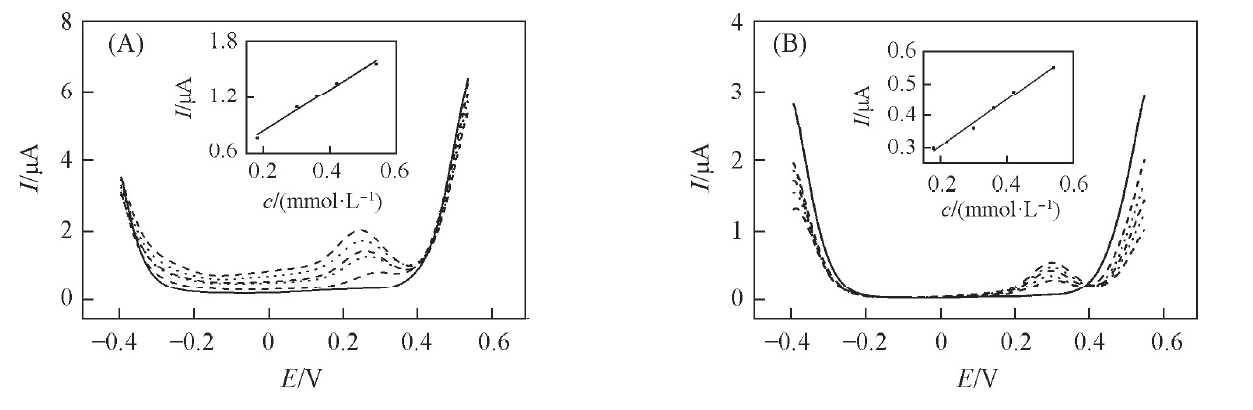
Fig.5 DPVs of the W/DCH interface modified by hybrid mesoporous membrane(A) and bare PET membrane(B) at increasing concentrations of phenazopyridine hydrochlorideFrom bottom to top, the concentrations of phenazopyridine hydrochloride are 0, 0.18, 0.3, 0.36, 0.42 and 0.54 mmol/L. Inset: the corresponding plots of peak current vs. the concentration of phenazopyridine hydrochloride in aqueous solution.
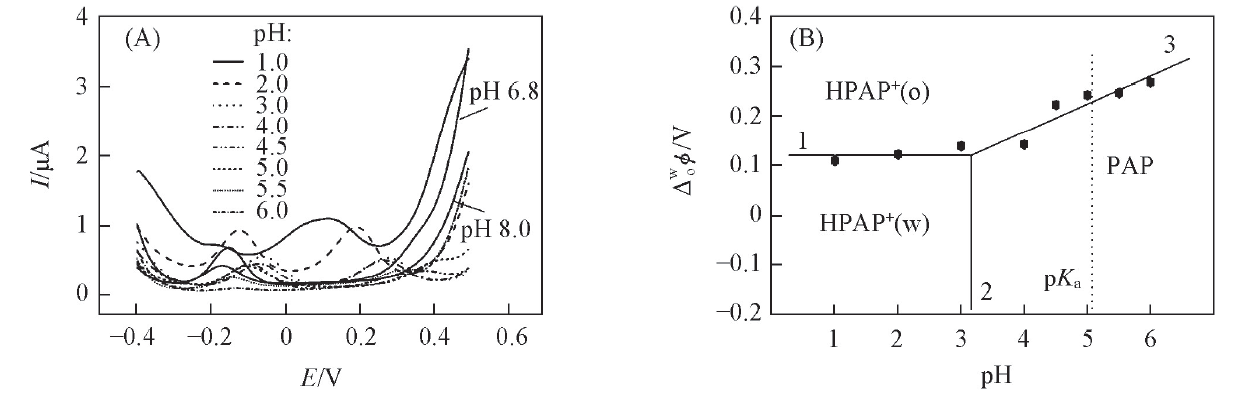
Fig.6 DPVs of the W/DCH interface modified by hybrid mesoporous membrane in presence of phenazopyridine hydrochloride and TBA+ at different pH values(A) and the corresponding plots of ionic partition diagram of PAP at W/DCH interface(B)The concentrations of phenazopyridine hydrochloride and TBA+ are 0.3 mmol/L, respectively. (B) The dotted line represents the pKa of PAP. Lines 1, 2, and 3 correspond to eqs.(3), (4) and (5), respectively.
| [1] | Hui J. Y., Harvey M. A., Johnston S. L., J. Obstet. Gynaecol. Can.,2009, 31(9), 845—849 |
| [2] | Tao Q., Chen J. M., Ma L., Lu T. B., Cryst. Growth. Des.,2012, 12, 3144—3152 |
| [3] | Tolba M. M., Salim M. M., J. Chromatogr. Sci.,2016, 54(5), 776—789 |
| [4] | Shang E., Xiang B., Liu G., Xie S. F., Wei W. Y., Lu J., Anal. Bioanal. Chem.,2005, 382, 216—222 |
| [5] | Suter D. M., Preynat S. O., Tirefort D., Feki A., Krause K. H., J. Cell. Mol. Med.,2009, 13(98), 3517—3527 |
| [6] | Reynolds E.F., Martindale: the Extra Pharmacopoeia, 30th Ed., the Pharmaceutical Press, London, 1993, 29 |
| [7] | Li T. Y., Wei J. M., Chin. J. Clin. Pharmacol.,2012, 28(11), 854—856 |
| (李天云, 魏敏吉. 中国临床药理学杂志,2012, 28(11), 854—856) | |
| [8] | Li P., Chen Q. H., Zhang Z., He J., Hu S. F., Chin. Hosp. Pharm. J.,2009, 29(1), 44—46 |
| (李鹏, 陈琴华, 张卓, 何婧, 胡士凤. 中国医院药学杂志,2009, 29(1), 44—46) | |
| [9] | Zhou H., Chen C. Y., Xie A. J., Spectroscopy and Spectral Analysis,2007, 27(9), 1830—1833 |
| (周宏, 陈昌云, 谢安建. 光谱学与光谱分析,2007, 27(9), 1830—1833) | |
| [10] | Pereira P. F., Silva W. P. D., Muñoz R. A. A., Richter E. M., J. Electroanal. Chem.,2016, 766, 87—93 |
| [11] | Ensafi A. A., Arashpour B., Rezaei B., Allafchian A. R., Colloid Surface B,2013, 111, 270—276 |
| [12] | Taei M., Hasanpour F., Movahedi M., Mohammadian S., Rsc. Adv.,2015, 5, 37431—37439 |
| [13] | Gu J., Qiao Y. H., Zhu X. Y., Yin X. H., Zhang X., Chen Y., Zhu Z. W., Shao Y. H., J. Electrochem.,2014, 20(3), 234—242 |
| (顾菁, 乔永辉, 朱新宇, 阴笑弘, 张欣, 陈烨, 朱志伟, 邵元华. 电化学,2014, 20(3), 234—242) | |
| [14] | Yuan Y., Su B., Shao Y. H., Chem. J. Chinese Universities,2001, 22(11), 1819—1823 |
| (袁艺, 苏彬, 邵元华. 高等学校化学学报,2001, 22(11), 1819—1823) | |
| [15] | Chen Y., Su B., Shao Y. H., Chem. J. Chinese Universities,2003, 24(10), 1834—1837 |
| (陈勇, 苏彬, 邵元华. 高等学校化学学报,2003, 24(10), 1834—1837) | |
| [16] | Pu G. Q., Zhang D. X., Mao X., Zhang Z., Wang H., Ning X. M., Lu X. Q., Anal. Chem.,2018, 90, 5272—5279 |
| [17] | Nagatani H., Fujisawa M., Imura H., Langmuir,2018, 34, 3237—3243 |
| [18] | Viada B. N., Juárez A. V., Gómez E. M. P., Fernández M. A., Yudi L. M., Electrochimica Acta,2018, 263, 499—507 |
| [19] | Langmaier J., Záliš S., Stanislav Samec Z., J. Electroanal. Chem.,2018, 815, 183—188 |
| [20] | Huang X., Xie L. Q., Lin X. Y., Su B., Anal. Chem.,2017, 89, 945—951 |
| [21] | Ribeiro J. A., Silva F., Pereira C. M., Anal. Chem.,2013, 85, 1582—1590 |
| [22] | Sairi M., Strutwolf J., Mitchell R. A., Silvester D. S., Arrigan D. W. M., Electrochimica Acta,2013, 101, 177—185 |
| [23] | Jiang X. H., Gao K., Hu D. P., Wang H. H., Bian S. J., Chen Y., Analyst,2015, 140, 2823—2833 |
| [24] | Zhang Y. H., Jiang T., Liu S. F., Yu Y. Q., Chen Y., Chem. J. Chinese Universities,2018, 39(4), 764—770 |
| (张烨桦, 姜涛, 刘书峰, 于雅倩, 陈勇. 高等学校化学学报,2018, 39(4), 764—770), 764—770) | |
| [25] | Liu S. J., Li Q., Shao Y. H., Chem. Soc. Rev.,2011, 40(5), 2236—2253 |
| [26] | Reymond F., Steyaert G., Carrupt P. A., Testa B., Girault H. H., J. Am. Chem. Soc.,1996, 118, 11951—11957 |
| [27] | Gobry V., Ulmeanu S., Reymond F., Bouchard G., Carrupt P. A., Testa B., Girault H. H., J. Am. Chem. Soc.,2001, 123, 10684—10690 |
| [28] | Chen Y., Bian S. J., Gao K., Cao Y. Y., Wu H. Q., Liu C. X., Jiang X. H., Sun X. L., J. Membrane Sci.,2014, 457, 9—18 |
| [29] | Gao K., Jiang X. H., Hu D. P., Bian S. J., Wang M., Chen Y., Chinese Chem. Lett.,2015, 26, 285—288 |
| [30] | Hu D. P., Wang H. H., Gao K., Jiang X. H., Wang M., Long Y. F., Chen Y., RSC Adv.,2014, 4, 57035—57040 |
| [31] | Lee H. J., Beattie P. D., Seddon B. J., Osborne M. D., Girault H. H., J. Electroanal. Chem.,1997, 440, 73—82 |
| [32] | Peng C., Li J. L., Li Z. W., Wu Z. J., Zhou D. Y., Mater. Design,2016, 111, 453—462 |
| [33] | Senthilkumar S., Dryfe R. A. W., Saraswathi R., Langmuir,2007, 23, 3455—3461 |
| [34] | Lam H. T., Pereira C. M., Roussel C., Carrupt P. A., Girault H. H., Anal. Chem.,2006, 78, 1503—1508 |
| [35] | Katano H., Senda M., Anal. Sci.,2001, 17, 1027—1029 |
| [1] | LI Yidi, TIAN Xiaochun, LI Junpeng, CHEN Lixiang, ZHAO Feng. Electron Transfer on the Semiconductor-microbe Interface and Its Environmental Application [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220089. |
| [2] | MA Yanrong, JIANG Shengnan, JIN Yan. Sensitive and Electrochemical Detection of Telomerase Activity Based on the Signal Amplification of Strand Displacement Reaction [J]. Chem. J. Chinese Universities, 2021, 42(3): 745. |
| [3] | YANG Pengfei, SHI Yuping, ZHANG Yanfeng. Large-scale Syntheses and Versatile Applications of Two-dimensional Metal Dichalcogenides [J]. Chem. J. Chinese Universities, 2021, 42(2): 504. |
| [4] | HAN Juntian,CUI Yaoxing,SU Zhijun,WU Yi,CHEN Liuping,XU Junhui. Two-Electron Storage Viologen for Aqueous Organic Redox Flow Batteries [J]. Chem. J. Chinese Universities, 2020, 41(5): 1035. |
| [5] | LIU Lu,WU Hanyue,LI Jing,SHE Lan. Tuning Microstructures of Iron-Nickel Alloy Catalysts for Efficient Oxygen Evolution Reaction † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1083. |
| [6] | HAN Fangjie, DAI Mengjiao, LIANG Zhishan, SONG Zhongqian, HAN Dongxue, NIU Li. Research Progress of Photoelectrochemical Technology Applied in Antioxidant Analysis † [J]. Chem. J. Chinese Universities, 2020, 41(4): 591. |
| [7] | FAN Hui, JIN Baokang. Investigation on Electrochemical Capture of CO2 by Quinone Derivatives Based on in situ FTIR Spectroelectrochemistry † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1847. |
| [8] | QIAO Xuejiao, LI Dan, CHENG Longjiu, JIN Baokang. Mechanism of Electrochemical Capture of CO2 via Redox Cycle of 2-Amino-3-chloro-1,4-naphthoquinone in BMIMBF4 [J]. Chem. J. Chinese Universities, 2019, 40(8): 1606. |
| [9] | LIANG Zhishan, NI Shuang, DAI Mengjiao, HAN Fangjie, HAN Lipeng, NIU Li, HAN Dongxue. Antioxidant Capacity Evaluation for Traditional Chinese Herbal Medicines Based on Sensitive g-C3N4/P25 Nanocomposite Photoelectrochemical Platform † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2081. |
| [10] | XU Suwen,YING Yilun,LONG Yitao. One-step Method for Fabrication of Closed Wireless Nanopore Electrode and Its Application on Single Nanoparticle Detection † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2075. |
| [11] | ZHU Yun,LI Dan,JIN Baokang. Study on Redox Mechanism of 2-Methyl-naphthoquinonein Dimethyl Sulfoxide and Acetonitrile† [J]. Chem. J. Chinese Universities, 2018, 39(9): 2025. |
| [12] | ZHANG Yehua, JIANG Tao, LIU Shufeng, YU Yaqian, CHEN Yong. Ion Transfer of Leucovorin Ion Across the Membrane-modified Liquid/Liquid Interface† [J]. Chem. J. Chinese Universities, 2018, 39(4): 764. |
| [13] | GAO Chengyao, TONG Jianhua, BIAN Chao, SUN Jizhou, LI Yang, WANG Jinfen, GONG Shun, HUI Yun, XIA Shanhong. Electroanalytical Sensing of Trace Cd(Ⅱ) Using in-situ Bismuth Modified Boron Doped Diamond Electrode† [J]. Chem. J. Chinese Universities, 2018, 39(3): 447. |
| [14] | WANG Yun,BEN Teng. Preparation and Electrochemical Performance of Seaweed-based Heteroatom-containing Carbon Materials† [J]. Chem. J. Chinese Universities, 2018, 39(12): 2627. |
| [15] | NI Jie, WEI Hengyong, BU Jinglong, LIU Huixing, CUI Yi, LÜ Dongfeng, WEI Yingna, ZHANG Lifang. Synthesis of Ordered Mesoporous TiN Powder via Ammonia Reduction Nitridation Reaction and Its Electrochemical Performance† [J]. Chem. J. Chinese Universities, 2017, 38(3): 355. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||