

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (1): 108.doi: 10.7503/cjcu20160452
• Physical Chemistry • Previous Articles Next Articles
ZHANG Bo1, HE Jun1, HUA Zhengshen1, WANG Xin1, PENG Huifen1,2,*( )
)
Received:2016-06-23
Online:2017-01-10
Published:2016-12-15
Contact:
PENG Huifen
E-mail:peng@hebut.edu.cn
Supported by:CLC Number:
TrendMD:
ZHANG Bo, HE Jun, HUA Zhengshen, WANG Xin, PENG Huifen. Effect of S
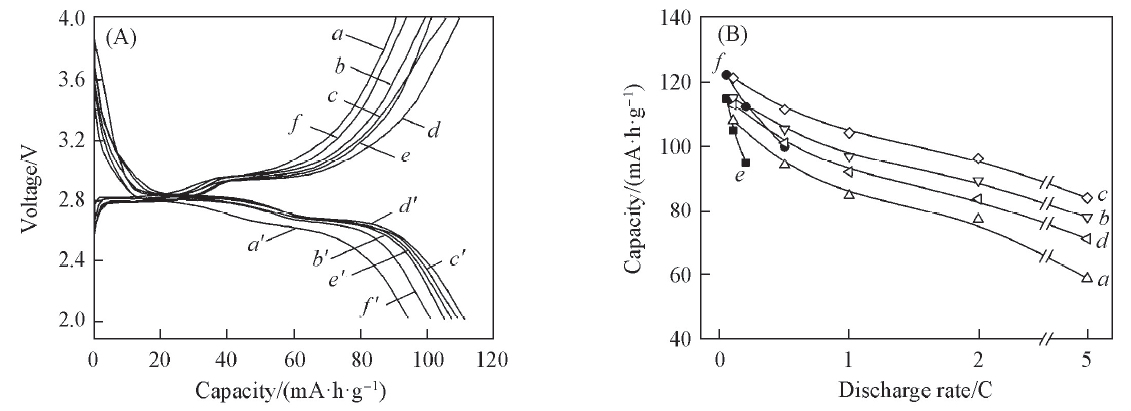
Fig.2 Initial charge-discharge curves at 0.5C(A) and initial discharge capacities at different rates(B) for Li3-xFe2(PO4)3-x(SO4)x (A) x: a, a'. 0; b, b'. 0.30; c, c'. 0.45; d, d'. 0.60; e, e'. 0.75; f, f'. 0.90. (B) x: a. 0; b. 0.30; c. 0.60; d. 0.90; e. Li3Fe2(PO4)3; f. Li2.8Fe1.8Ti0.2(PO4)3.
| x | Rct/Ω | σ/(Ω·cm2·s-1/2) | 1012D/(cm2·s-1) | x | Rct/Ω | σ/(Ω·cm2·s-1/2) | 1012D/(cm2·s-1) |
|---|---|---|---|---|---|---|---|
| 0 | 196.0 | 97.8 | 3.97 | 0.60 | 103.6 | 31.9 | 37.30 |
| 0.30 | 137.6 | 50.2 | 13.90 | 0.75 | 127.8 | 75.8 | 6.60 |
| 0.45 | 117.7 | 42.3 | 21.20 | 0.90 | 170.5 | 84.3 | 5.34 |
Table 1 Charge transfer resistance(Rct), Warburg coefficients(σ) and Li+ diffusion coefficients(D) of the Li3-xFe2(PO4)3-x(SO4)x samples
| x | Rct/Ω | σ/(Ω·cm2·s-1/2) | 1012D/(cm2·s-1) | x | Rct/Ω | σ/(Ω·cm2·s-1/2) | 1012D/(cm2·s-1) |
|---|---|---|---|---|---|---|---|
| 0 | 196.0 | 97.8 | 3.97 | 0.60 | 103.6 | 31.9 | 37.30 |
| 0.30 | 137.6 | 50.2 | 13.90 | 0.75 | 127.8 | 75.8 | 6.60 |
| 0.45 | 117.7 | 42.3 | 21.20 | 0.90 | 170.5 | 84.3 | 5.34 |
| [1] | Padhi A. K., Nanjundaswamy K. S., Goodenough J. B., J. Electrochem. Soc., 1997, 144(4), 1188—1194 |
| [2] | Bakenov Z., Taniguchi I., J. Power Sources,2010, 195(21), 7445—7451 |
| [3] | D’Yvoire F., Pintard-Screpel M., Bretey E., Rochere M., Solid State Ionics,1983, 9, 851—857 |
| [4] | Mi C. H., Cao Y. X., Zhang X. G., Zhao X. B., Li H. L., Powder Technol., 2008, 181(3), 301—306 |
| [5] | Yamada A., Koizumi H., Nishimura S., Sonoyama N., Kanno R., Yonemura M., Nakamura T., Kobayashi Y., Nat. Mater., 2006, 5(5), 357—360 |
| [6] | Butt G., Sammes N., Tompsett G., Smirnova A., Yamamoto O., J. Power Sources,2004, 134(1), 72—79 |
| [7] | Bykov A. B., Chirkin A. P., Demyanets L. N., Doronin S. N., Genkina E. A., Ivanov-shits A. K., Kondratyuk I. P., Maksimov O. K., Muradyan L. N., Simonov V. I., Timofeeva V. A., Solid State Ionics,1990, 38(1), 31—52 |
| [8] | Ait Salah A., Jozwiak P., Garbarczyk J., Benkhouja K., Zaghib K., Gendron F., Julien C. M., J. Power Sources,2005, 140(2), 370—375 |
| [9] | Ivanov-Schitz A. K., Nistuk A. V., Chaban N. G., Solid State Ionics,2001, 139(1/2), 153—157 |
| [10] | Karami H., Taala F., J. Power Sources,2011, 196(15), 6400—6411 |
| [11] | Morcretter M., Werm C., Masquelier C., Solid State Science,2002, 4(2), 239—246 |
| [12] | Song C. H., Sun J. K., Huang F. Q., Liu Z. Q., He P. G., Functional Mater., 2009, 40(4), 604—607 |
| (宋翠环, 孙军康, 黄富强, 刘战强, 何品刚. 功能材料, 2009, 40(4), 604—607) | |
| [13] | Sun J. K., Huang F. Q., Wang Y. M., Shan Z. C., Liu Z. Q., Liu M. L., Xia Y. J., Li K. Q., J. Alloys Compd., 2009, 469(1), 327—331 |
| [14] | Plylahan N., Vidal-Abarca C., Lavela P., Tirado J. L., Electrochim. Acta,2012, 62(1), 124—131 |
| [15] | Liu Z. Q., Huang F. Q., Sun J. K., Mater. Sci. Eng. B,2011, 176(18), 1313—1316 |
| [16] | Hagh N. M., Amatucci G. G., J. Power Sources,2014, 256(256), 457—469 |
| [17] | Bensch W., Bredow T., Ebert H., Heitjans P., Indris S., Mankovsky S., Wilkening M., Progress in Solid State Chem., 2009, 37(2), 206—225 |
| [18] | Ma L., Chen H.J., Basic Chemistry, 2nd Edition, Chemical Industry Press, Beijing, 2011, 478 |
| (马荔, 陈虹锦. 基础化学, 第2版, 北京: 化学工业出版社, 2011, 478) | |
| [19] | Padhi A. K., Manivannan V., Goodenough J. B., J. Electrochem. Soc., 1998, 145(5), 1518—1520 |
| [20] | Geng S. X., Yang Y. G., Zhang Y. G., Ding W., Wang X., Peng H. F., Bakenov Z., Electrochim. Acta,2015, 176, 327—333 |
| [21] | Nanjundaswamya K. S., Padhi A. K., Goodenough J. B., Okada S., Ohtsuka H., Arai H., Yamaki J., Solid State Ionics,1996, 92(1/2), 1—10 |
| [22] | Masquelier C., Padhi A. K., Nanjundaswamy K. S., Goodenough J. B., J. Solid State Chem., 1998, 135(2), 228—234 |
| [23] | Wu P., Du N., Zhang H., Yang D. R., Lu T. H., Chem. J. Chinese Universities,2013, 34(3), 668—673 |
| (吴平, 杜宁, 张辉, 杨德仁, 陆天虹. 高等学校化学学报, 2013, 34(3), 668—673) | |
| [24] | Luo P. W., Yu J. G., Shi Z. Q., Huang H., Liu L., Zhao Y. N., Li G. D., Zou Y. C., Chem. Res. Chinese Universities,2012, 28(5), 780—783 |
| [25] | Liu H., Cao Q., Fu L. J., Li C., Wu Y. P., Wu H. Q., Electrochem. Commun., 2006, 8(10), 1553—1557 |
| [26] | Liu H., Li C., Zhang H. P., Fu Y. P., Wu H. Q., J. Power Sources,2006, 159(1), 717—720 |
| [27] | Liu H. P., Bi S. F., Wen G. W., Teng X. G., Gao P., Ni Z. J., Zhu Y. M., J. Alloys Compd., 2012, 43(543), 99—104 |
| [1] | ZHANG Shiyu, HE Runhe, LI Yongbing, WEI Shijun, ZHANG Xingxiang. Fabrication of Lithium-sulfur Battery Cathode with Radiation Crosslinked Low Molecular Weight of Polyacrylonitrile and the Mechanism of Sulfur Storage [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210632. |
| [2] | GAO Xiaole, WANG Jiaxin, LI Zhifang, LI Yanchun, YANG Donghua. Synthesis of NiOx-ZSM-5 Composite Materials and Its Electrocatalytic Hydrogen Evolution Performance in Microbial Electrolysis Cell [J]. Chem. J. Chinese Universities, 2021, 42(9): 2886. |
| [3] | BAO Junquan, ZHENG Shibing, YUAN Xuming, SHI Jinqiang, SUN Tianjiang, LIANG Jing. An Organic Salt PTO(KPD)2 with Enhanced Performance as a Cathode Material in Lithium-ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(9): 2911. |
| [4] | FAN Xiaoyong, WU Yan, SUN Ruibo, GOU Lei, LI Donglin. Construction and Zn Storage Performance of Three Dimensional Porous MnOx@In2O3 Cubes [J]. Chem. J. Chinese Universities, 2021, 42(6): 1816. |
| [5] | WANG Yimeng, LIU Kai, WANG Baoguo. Coating Strategies of Ni-rich Layered Cathode in LIBs [J]. Chem. J. Chinese Universities, 2021, 42(5): 1514. |
| [6] | WANG Renheng, XIAO Zhe, LI Yan, SUN Yiling, FAN Shuting, ZHENG Junchao, QIAN Zhengfang, HE Zhenjiang. Synthesis of Li2FeP2O7 Cathode Material at Different Temperatures and Its Electrochemical Performance for Lithium Ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(4): 1299. |
| [7] | ZHANG Huishuang, GAO Yanxiao, WANG Qiuxian, LI Xiangnan, LIU Wenfeng, YANG Shuting. High-low Temperature Properties of Ni-rich LiNi0.6Co0.2Mn0.2O2 Cathode Material by Hydrothermal Synthesis with CTAB Assisted [J]. Chem. J. Chinese Universities, 2021, 42(3): 819. |
| [8] | HUANG Yongfeng, HUANG Wenting, LIU Wenbao, LIU Yuefeng, LIU Wei, XU Chengjun. Mechanism of Storage and Capacity Attenuation of V2O5 as Cathode of Zinc-ion Battery [J]. Chem. J. Chinese Universities, 2020, 41(8): 1859. |
| [9] | LU Di,ZHENG Chunman,CHEN Yufang,LI Yujie,ZHANG Hongmei. Synthesis of Li-rich Layers/Spinel/Carbon Composite Cathode Materials with Phenol Formaldehyde Resin and Its Electrochemical Performance† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1684. |
| [10] | BAI Yanqun,WANG Cunguo,LI Xue,FAN Wenqi,SONG Penghao,GU Yuanchun,LIU Faqian,LIU Guangye. Preparation and Electrochemical Properties of S@C Composite Material with High Capacity and Ordered Alignment of Channels † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1306. |
| [11] | DENG Anqiang,LUO Yongchun,XIA Yuanhua,PENG Sihui,MA Weiqin,ZHAO Xudong,YANG Yang,HOU Xiaodong. Effect of Annealing Treatment on Structure and Electrochemical Properties of New Mg-free Y0.7La0.3Ni3.25Al0.1Mn0.15 Hydrogen Storage Alloys † [J]. Chem. J. Chinese Universities, 2020, 41(1): 145. |
| [12] | LI Xin, CHEN Liang, MA Xiaotao, ZHANG Ding, XU Shoudong, ZHOU Xianxian, DUAN Donghong, LIU Shibin. Preparation of V2O3 Hollow Spheres for Lithium Sulfur Batteries † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1972. |
| [13] | YANG Jinge, LI Yujie, LU Di, CHEN Yufang, SUN Weiwei, ZHENG Chunman. Morphology Control and Lithium Storage Performance of Micro/nano Li-rich Cathode Material† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1495. |
| [14] | YAO Fengnan,LI Yu,FENG Wei. Synthesis and Electrochemical Performance of Carbon-coated FeF2 Nanocomposite† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1418. |
| [15] | MA Dongwei,TIAN Runsai,LIU Zhenjiang,FENG Yuanyuan,DING Hongyu,FENG Jijun. Microwave-assisted Synthesis and Electrochemical Performance of Na-Doped Cathode Materials Li2-xNaxMnSiO4/C† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1280. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||