

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (7): 1320.doi: 10.7503/cjcu20160092
• Organic Chemistry • Previous Articles Next Articles
JIAO Jing1, YANG Xue1, JIN Lanna1, GAO Jian1, ZHOU Yang1, XIAO Yazhong3,*( ), ZHANG Yingjiu1,2,*(
), ZHANG Yingjiu1,2,*( )
)
Received:2016-02-06
Online:2016-07-10
Published:2016-06-17
Contact:
XIAO Yazhong,ZHANG Yingjiu
E-mail:yazxiao@ahu.edu.cn;yingjiu@jlu.edu.cn
CLC Number:
TrendMD:
JIAO Jing, YANG Xue, JIN Lanna, GAO Jian, ZHOU Yang, XIAO Yazhong, ZHANG Yingjiu. Conservative and Variability of the Important Functional Sites in a Laccase from Bacillus Subtilis†[J]. Chem. J. Chinese Universities, 2016, 37(7): 1320.
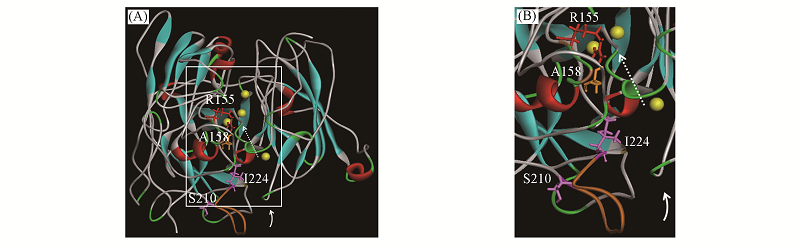
Fig.1 3D structure of laccase CARFig.1(B) is an enlarged view of the white box in Fig.1(A). Balls: copper ions; solid arrows: the entrance of substrates; dashed arrows: electron transfer from the mononuclear centre to the trinuclear centre of CAR; orange peptide fragment: pro-rich peptide fragment.
| Primer | Sequence(5'→3') | Primer | Sequence(5'→3') |
|---|---|---|---|
| CAHS | CGATCACGCCATGGCGCTC | CAHEGS | TTATCCGGGCGCACCGGAA |
| CAHA | CCATGGCGTGATCGTGATACCAC | CAHEGA | TTCCGGTGCGCCCGGATAA |
| CAHES | TCACGCCATGGAGCTC | CAHEGFS | AATCCTTCATTCGTTC |
| CAHEA | TGAGCTCCATGGCGTGATC | CAHEGFA | GAACGAATGAAGGATT |
Table 1 Designed primers
| Primer | Sequence(5'→3') | Primer | Sequence(5'→3') |
|---|---|---|---|
| CAHS | CGATCACGCCATGGCGCTC | CAHEGS | TTATCCGGGCGCACCGGAA |
| CAHA | CCATGGCGTGATCGTGATACCAC | CAHEGA | TTCCGGTGCGCCCGGATAA |
| CAHES | TCACGCCATGGAGCTC | CAHEGFS | AATCCTTCATTCGTTC |
| CAHEA | TGAGCTCCATGGCGTGATC | CAHEGFA | GAACGAATGAAGGATT |
| Dye | Mw/Da | c/(mg·L-1) | λmax/nm |
|---|---|---|---|
| Indigo carmine | 466.37 | 40 | 610 |
| Crystal violet | 407.98 | 5 | 590 |
| Gongo red | 696.67 | 10 | 595 |
| Methyl red | 269.30 | 50 | 410 |
| Remazol brilliant blue R(RBBR) | 626.54 | 100 | 591 |
Table 2 Final concentration and maximum absorbance wavelengths of the tested dyes
| Dye | Mw/Da | c/(mg·L-1) | λmax/nm |
|---|---|---|---|
| Indigo carmine | 466.37 | 40 | 610 |
| Crystal violet | 407.98 | 5 | 590 |
| Gongo red | 696.67 | 10 | 595 |
| Methyl red | 269.30 | 50 | 410 |
| Remazol brilliant blue R(RBBR) | 626.54 | 100 | 591 |
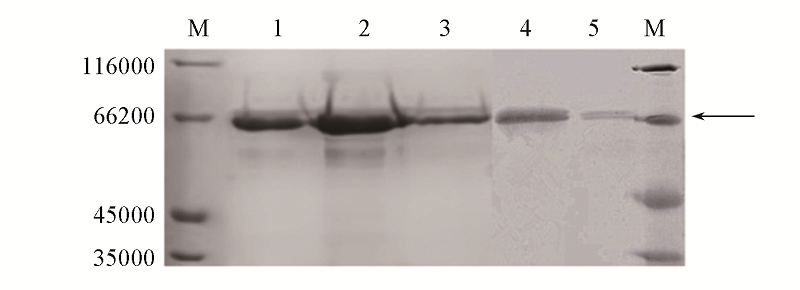
Fig.2 SDS-PAGE analysis of the purified laccases Lane M: protein markers; lanes 1—3: purified laccase CAHEG,CAH and CAHEGF; lanes 4 and 5: purified laccase CAHE.
| Enzyme | Specific activity/ (U·mg-1) | Oxidation-reduction potential/V | Enzyme | Specific activity/ (U·mg-1) | Oxidation-reduction potential/V |
|---|---|---|---|---|---|
| CAR | 17.1 | 0.320 | CAHEG | 99.0 | 0.395 |
| CAH | 220.4 | 0.420 | CAHEGF | 67.7 | 0.345 |
| CAHE | 70.2 | 0.385 |
Table 3 Specific activities and oxidation-reduction potentials of the wild type and mutant laccases
| Enzyme | Specific activity/ (U·mg-1) | Oxidation-reduction potential/V | Enzyme | Specific activity/ (U·mg-1) | Oxidation-reduction potential/V |
|---|---|---|---|---|---|
| CAR | 17.1 | 0.320 | CAHEG | 99.0 | 0.395 |
| CAH | 220.4 | 0.420 | CAHEGF | 67.7 | 0.345 |
| CAHE | 70.2 | 0.385 |
| Enzyme | Km/(mmol·L-1) | kcat/s-1 | kcat· |
|---|---|---|---|
| CAR | 0.17 | 26.0 | 153.0 |
| CAHE | 0.13 | 34.0 | 263.0 |
| CAHEGF | 0.14 | 34.0 | 243.0 |
| CAHEG | 0.12 | 35.0 | 292.0 |
| CAH | 0.10 | 85.0 | 850.0 |
Table 4 Kinetic parameters of the wild type and mutant laccases
| Enzyme | Km/(mmol·L-1) | kcat/s-1 | kcat· |
|---|---|---|---|
| CAR | 0.17 | 26.0 | 153.0 |
| CAHE | 0.13 | 34.0 | 263.0 |
| CAHEGF | 0.14 | 34.0 | 243.0 |
| CAHEG | 0.12 | 35.0 | 292.0 |
| CAH | 0.10 | 85.0 | 850.0 |
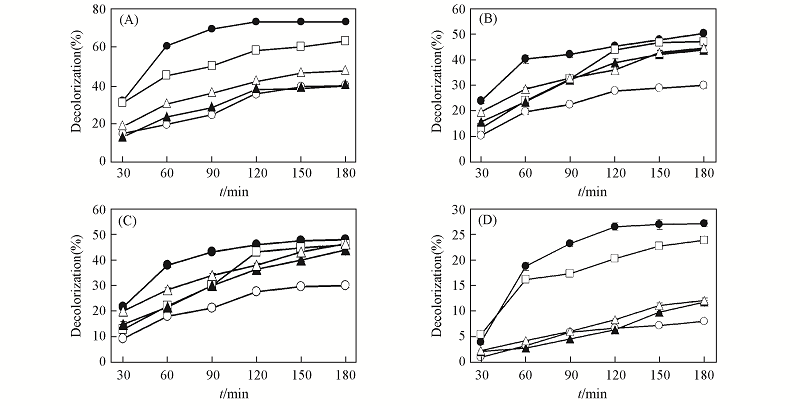
Fig.4 Decolorization of several dyes by the wild type and mutant laccases(A) CAH; (B) CAHEG; (C) CAHEGF; (D) CAR. ● Indigo carmine; □ crystal violet; △ gongo red; ▲ methyl red; ○ RBBR.
| [1] | Gao J., Guan K. X., Jiao J., Jiang W. Z., Zhang Y. J., Journal of Molecular Catalysis,2014, 28(2), 188—196(高键, 关可兴, 焦晶, 姜文洙, 张应玖. 分子催化, 2014, 28(2), 188—196) |
| [2] | Giardina P., Sannia G., Cell Mol. Life Sci. J., 2015, 72(5), 855—856 |
| [3] | Shraddha, Shekher R., Sehgal S., Kamthania M., Kumar A.,Enzyme Res., 2011, 217861 |
| [4] | Moccelini S. K., Franzoi A. C., Vieira I. C., Dupont J., Scheeren C. W., Biosens. Bioelectron., 2011, 26(8), 3549—3554 |
| [5] | Guan Z. B., Shui Y., Song C. M., Zhang N., Cai Y. J., Liao X. R., Environ. Sci. Pollut. Res. Int., 2015, 22(12), 9515—9523 |
| [6] | Chandra R., Chowdhary P., Environ. Sci. Processes Impacts., 2015, 17(2), 326—342 |
| [7] | Virk A. P., Sharma P., Capalash N., Biotechnology Prog., 2012, 28(1), 21—32 |
| [8] | Osma J.F., Toca-Herrera J. L., Rodríguez-Couto S.,Enzyme Res., 2010, 918761 |
| [9] | Abdel-Hamid A. M., Solbiati J. O., Cann I. K., Adv. Appl. Microbiol., 2013, 82, 1—28 |
| [10] | Pollegioni L., Tonin F., Rosini E., FEBS J. Lignin-degrading Enzymes,2015, 282(7), 1190—1213 |
| [11] | Wang J., Feng J., Jia W., Chang S., Li S., Li Y., Biotechnol. Biofuels,2015, 8, 145 |
| [12] | Roth S., Spiess A. C., Bioprocess Biosyst. Eng., 2015, 38(12), 2285—2313 |
| [13] | Reiss R., Ihssen J., Thöny-Meyer L., BMC Biotechnol., 2011, 11, 9 |
| [14] | Mollania N., Khajeh K., Ranjbar B., Enzyme Microb. Technol., 2013, 52(6/7), 325—330 |
| [15] | Mohammadian M., Fathi-Roudsari M., Mollania N., J. Ind. Microbiology Biotechnol. J., 2010, 37(8), 863—869 |
| [16] | Pezzella C., Guarino L., Piscitelli A., Cell Mol. Life Sci., 2015, 72(5), 923—940 |
| [17] | Kudanga T., le Roes-Hill M., Appl. Microbiol. Biotechnol., 2014, 98(15), 6525—6542 |
| [18] | Sitarz A. K., Mikkelsen J. D., Meyer A. S., Crit. Rev. Biotechnol., 2016, 36(1), 70—86 |
| [19] | Gündüz Ergün B., Çalık P., Bioprocess Biosyst. Eng., 2015, 39(1), 1—36 |
| [20] | Hakulinen N., Rouvinen J., Cell Mol. Life Sci., 2015, 72(5), 857—868 |
| [21] | Mate D. M., Alcalde M., Biotechnol. Adv., 2015, 33(1), 25—40 |
| [22] | Jia H., Lee F. S., Farinas E. T., ACS Comb. Sci., 2014, 16(12), 665—669 |
| [23] | Gupta N., Farinas E. T., Protein Eng. Des. Sel., 2010, 23(8), 679—682 |
| [24] | Xia Y., Cao H., Zhang Y. J., Chem. J. Chinese Universities,2015, 36(2), 330—335(夏莹, 曹昊, 张应玖. 高等学校化学学报, 2015, 36(2), 330—335) |
| [25] | Bourbonnais R., Paice M. G., FEBS Lett., 1990, 267(1), 99—102 |
| [26] | Enguita F. J., Martins L. O., Henriques A. O., Carrondo M. A., J. Biol. Chem., 2003, 278(21), 19416—19425 |
| [1] | WANG Sicong, PANG Beibei, LIU Xiaokang, DING Tao, YAO Tao. Application of XAFS Technique in Single-atom Electrocatalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220487. |
| [2] | YANG Jingyi, LI Qinghe, QIAO Botao. Synergistic Catalysis Between Ir Single Atoms and Nanoparticles for N2O Decomposition [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220388. |
| [3] | LIN Gaoxin, WANG Jiacheng. Progress and Perspective on Molybdenum Disulfide with Single-atom Doping Toward Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220321. |
| [4] | TANG Quanjun, LIU Yingxin, MENG Rongwei, ZHANG Ruotian, LING Guowei, ZHANG Chen. Application of Single-atom Catalysis in Marine Energy [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220324. |
| [5] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [6] | YAO Qing, YU Zhiyong, HUANG Xiaoqing. Progress in Synthesis and Energy-related Electrocatalysis of Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220323. |
| [7] | JIANG Bowen, CHEN Jingxuan, CHENG Yonghua, SANG Wei, KOU Zongkui. Recent Progress of Single-atom Materials in Electrochemical Biosensing [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220334. |
| [8] | LIN Zhi, PENG Zhiming, HE Weiqing, SHEN Shaohua. Single-atom and Cluster Photocatalysis: Competition and Cooperation [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220312. |
| [9] | TENG Zhenyuan, ZHANG Qitao, SU Chenliang. Charge Separation and Surface Reaction Mechanisms for Polymeric Single-atom Photocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220325. |
| [10] | YANG Jingyi, SHI Siqi, PENG Huaitao, YANG Qihao, CHEN Liang. Integration of Atomically Dispersed Ga Sites with C3N4 Nanosheets for Efficient Photo-driven CO2 Cycloaddition [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220349. |
| [11] | WANG Ruyue, WEI Hehe, HUANG Kai, WU Hui. Freezing Synthesis for Single Atom Materials [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220428. |
| [12] | WANG Xintian, LI Pan, CAO Yue, HONG Wenhao, GENG Zhongxuan, AN Zhiyang, WANG Haoyu, WANG Hua, SUN Bin, ZHU Wenlei, ZHOU Yang. Techno-economic Analysis and Industrial Application Prospects of Single-atom Materials in CO2 Catalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220347. |
| [13] | HUANG Qiuhong, LI Wenjun, LI Xin. Organocatalytic Enantioselective Mannich-type Addition of 5H-Oxazol-4-ones to Isatin Derived Ketimines [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220131. |
| [14] | TAN Yan, YU Shen, LYU Jiamin, LIU Zhan, SUN Minghui, CHEN Lihua, SU Baolian. Efficient Preparation of Mesoporous γ-Al2O3 Microspheres and Performance of Pd-loaded Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220133. |
| [15] | WU Yushuai, SHANG Yingxu, JIANG Qiao, DING Baoquan. Research Progress of Controllable Self-assembled DNA Origami Structure as Drug Carrier [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220179. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||