

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (11): 2271.doi: 10.7503/cjcu20150651
• Physical Chemistry • Previous Articles Next Articles
WANG Mei1,2, WANG Jun1, BU Yuxiang1,*( )
)
Received:2015-08-14
Online:2015-11-10
Published:2015-10-21
Contact:
BU Yuxiang
E-mail:byx@sdu.edu.cn
CLC Number:
TrendMD:
WANG Mei, WANG Jun, BU Yuxiang. Metastable Hydrogen-bonds Featuring Negative Dissociation Energies in Protein-bound DNA in Hole Migration[J]. Chem. J. Chinese Universities, 2015, 36(11): 2271.
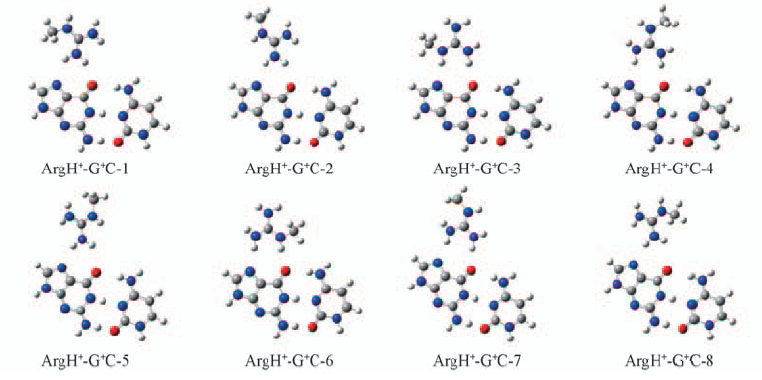
Fig.2 Optimized geometrical structures of eight complexes ArgH+-G+C-n(n=1—8) in which positive charged arginine residue resides in the major-groove face of DNA base pair
| Complex | R(N—H…N) | R(N—H…O) | RE | ΔE | ΔEBSSE | DE | AIP |
|---|---|---|---|---|---|---|---|
| ArgH+-G+C-1 | 0.2354 | 0.2123 | 11.25 | -118.95 | -120.79 | 5.56 | 911.19 |
| ArgH+-G+C-2 | 0.2203 | 0.2290 | 9.75 | -117.45 | -119.04 | 4.81 | 908.51 |
| ArgH+-G+C-3 | 0.2150 | 0.2003 | 3.01 | -110.67 | -113.22 | 5.98 | 919.22 |
| ArgH+-G+C-4 | 0.2101 | 0.2017 | 0.38 | -108.07 | -110.46 | 6.07 | 918.05 |
| ArgH+-G+C-5 | 0.2153 | 0.2429 | 9.87 | -117.53 | -119.12 | 4.56 | 920.73 |
| ArgH+-G+C-6 | 0.2101 | 0.2050 | 0 | -111.21 | -113.72 | 6.40 | 937.09 |
| ArgH+-G+C-7 | 0.2104 | 0.2011 | 3.51 | -107.70 | -110.08 | 5.94 | 934.50 |
| ArgH+-G+C-8 | 0.2117 | 0.2687 | 11.21 | -118.87 | -120.67 | 4.48 | 922.99 |
Table 1 Lengths(nm) of two hydrogen bonds(N—H…N7 H-bond and N—H…O6 H-bond) and dissociation energies(ΔE) of eight complexes ArgH+-G+C-n(n=1—8)*
| Complex | R(N—H…N) | R(N—H…O) | RE | ΔE | ΔEBSSE | DE | AIP |
|---|---|---|---|---|---|---|---|
| ArgH+-G+C-1 | 0.2354 | 0.2123 | 11.25 | -118.95 | -120.79 | 5.56 | 911.19 |
| ArgH+-G+C-2 | 0.2203 | 0.2290 | 9.75 | -117.45 | -119.04 | 4.81 | 908.51 |
| ArgH+-G+C-3 | 0.2150 | 0.2003 | 3.01 | -110.67 | -113.22 | 5.98 | 919.22 |
| ArgH+-G+C-4 | 0.2101 | 0.2017 | 0.38 | -108.07 | -110.46 | 6.07 | 918.05 |
| ArgH+-G+C-5 | 0.2153 | 0.2429 | 9.87 | -117.53 | -119.12 | 4.56 | 920.73 |
| ArgH+-G+C-6 | 0.2101 | 0.2050 | 0 | -111.21 | -113.72 | 6.40 | 937.09 |
| ArgH+-G+C-7 | 0.2104 | 0.2011 | 3.51 | -107.70 | -110.08 | 5.94 | 934.50 |
| ArgH+-G+C-8 | 0.2117 | 0.2687 | 11.21 | -118.87 | -120.67 | 4.48 | 922.99 |
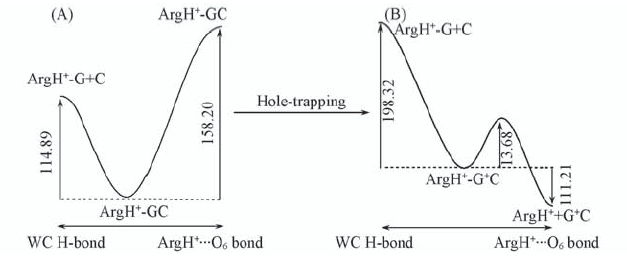
Fig.3 Schematic profiles of PES along WC and Hoogsteen H-bond dissociation coordinates of trimer complex units in their initial(ArgH+-GC) and oxidized(ArgH+-G+C) The corresponding dissociation are also shown. All the energies are expressed in kJ/mol.
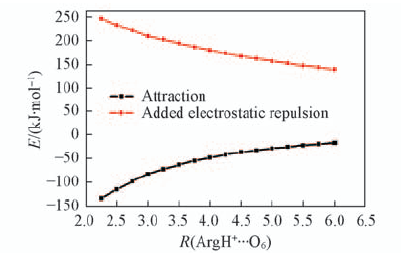
Fig.7 Effects of R(ArgH+…O6)(the distance between ArgH+ and O6 atom) on attraction interaction between ArgH+ and GC in ArgH+-GC, and added electrostatic repulsion upon one-electron oxidation The data for this figure are given in Table S4(see the Electronic Supporting Information of this paper).
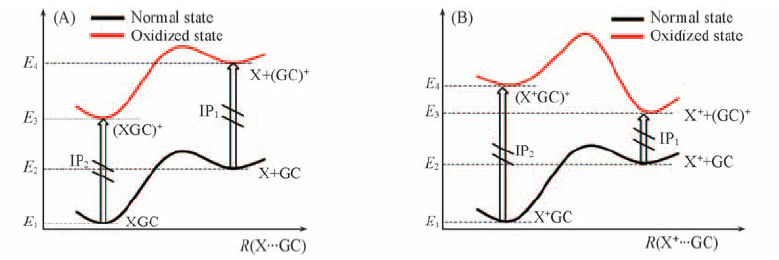
Fig.8 Potential energy surfaces of complexes XGC, X+GC(A) and their one-electron oxidized derivatives(XGC)+ and (X+GC)+(B) when the monomer X(or X+) is separated from GC and G+C, respectively En is the corresponding energy; the corresponding IPs are also shown.
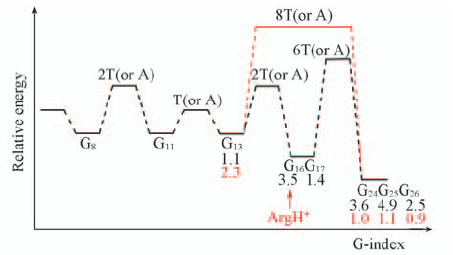
Fig.10 Schematic representation of potential energy landscapes through duplex DNA oligomers The DNA sequence is shown in Fig.S1. The “G”, “GG” and “GGG” denote an “isolated” guanine and two or three adjacent guanines, respectively. The “T” or “A” or “C” separates G or GG steps. The X-axis(“G-index”) indicates the position of guanines, GG steps, and GGG triplet along the oligomer and the energy barriers may be one base pair or some base pairs. The numbers in black and red indicate the band intensity relative to that of G24 in the protein-bound DNA duplex in the absence and presence of BamHI.
| [1] | Burrows C. J., Muller J. G., Chem. Rev., 1998, 98, 1109—1152 |
| [2] | LePage F., Guy A., Cadet J., Sarasin A., Gentil A., Nucleic Acids Res., 1998, 26, 1276—1281 |
| [3] | Burrows C. J., Muller J. G., Chem. Rev., 1998, 98, 1109—1152 |
| [4] | Hirakawa K., Ota K., Hirayama J., Oikawa S., Kawanishi S., Chem. Res. Toxicol., 2014, 27(4), 649—655 |
| [5] | Melvin T., Botchway S., Parker A. W., O’Neill P. J., Chem. Soc. Chem. Commun., 1995, 5, 653—654 |
| [6] | Steenken S., Jovanovic S. V., J. Am. Chem. Soc., 1997, 119, 617—618 |
| [7] | Jortner J., Bixon M., Langenbacher T., Michael-Beyerle M. E., Proc. Natl. Acad. Sci., 1998, 95, 12759—12765 |
| [8] | Berlin Y. A., Burin A. L., Ratner M. A., J. Am. Chem. Soc. ,2000, 122, 10903—10909 |
| [9] | Schuster G. B., Acc. Chem. Res. 2000, 33, 253—260 |
| [10] | Lewis F. D., Letsinger R. L., Wasielewski M. R., Acc. Chem. Res., 2001, 34, 159—170 |
| [11] | Wang J., Sun L.X., Bu Y. X., J. Phys. Chem. B, 2010, 114, 1144—1147 |
| [12] | Echols H., Science, 1986, 233, 1050—1056 |
| [13] | Kathuria P., Sharma P., Abendong M. N., Wetmore S. D., Biochemistry, 2015, 54(15), 2414—2428 |
| [14] | Qin P. H., Lü W. C., Qin W., Zhang W., Xie H., Chem. Res. Chinese Universities, 2014, 30(1), 125—129 |
| [15] | Corbella M., Voityuk A. A., Curutchet.C., J. Phys. Chem. Lett., 2015, 6(18), 3749—3753 |
| [16] | Paillard G., Lavery R., Structure, 2004, 12, 113—122 |
| [17] | Peters M., Rozas I., Alkorta I., Elguero J., J. Phys. Chem. B, 2003, 107, 323—330 |
| [18] | Wintjens R., Lievin J., Rooman M., Buisine E., J. Mol. Biol., 2000, 302(2), 395—410 |
| [19] | Warner D. R., Weinstein L. S., Proc. Natl. Acad. Sci., 1999, 96, 4268—4272 |
| [20] | Bond P. J., Guy A. T., Heron A. J., Bayley H., Khalid S., Biochemistry, 2011, 50(18), 3777—3783 |
| [21] | Jantz D., Berg J. M., J. Am. Chem. Soc., 2003, 125, 4960—4961 |
| [22] | Cheng A. C., Chen W. W., Fuhrmann C. N., Frankel A. D., J. Mol. Biol., 2003, 327, 781—796 |
| [23] | Allers J., Shamoo Y., J. Mol. Biol., 2001, 311, 75—86 |
| [24] | Davey C. A., Sargent D. F., Luger K., Maeder A. W., Richmond T. J., J. Mol. Biol., 2002, 319, 1097—1113 |
| [25] | Widom J., Annu. Rev. Biophys. Biomol. Struct., 1998, 27, 285—327 |
| [26] | Harp J. M., Hanson B. L., Tim D. E., Bunick G. J., Biol. Crystallogr., 2000, 56, 1513—1534 |
| [27] | Newman M., Strzelecka T., Dorner L. F., Schildkraut I., Aggarwal A. K., Science, 1995, 269, 656—663 |
| [28] | Rajski S. R., Barton J. K., Biochemistry, 2001, 40, 5556—5564 |
| [29] | Nunez M. E., Noyes K. T., Barton J. K., Chem. Biol., 2002, 9, 403—406 |
| [30] | Voityuk A. A., Davis W. B., J. Phys. Chem. B, 2007, 111, 2976—2985 |
| [31] | Bjorklund C. C., Davis W. B., Nucleic Acids Res., 2006, 34, 1836—1847 |
| [32] | Nakatani K., Dohno C., Saito I., J. Am. Chem. Soc., 2002, 124(24), 6802—6803 |
| [33] | Becke A. D., J. Chem. Phys., 1993, 98, 1372—1377 |
| [34] | Lee C., Yang W., Parr R. G., Phys. Rev. B, 1988, 37, 785—789 |
| [35] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Zakrzewski V. G., Montgomery J. A., Stratmann Jr., R.E., Burant J. C., Dapprich S., Millam J. M., Daniels A. D., Kudin K. N., Strain M. C., Farkas O., Tomasi J., Barone V., Cossi M., Cammi R., Mennucci B., Pomelli C., Adamo C., Clifford S., Ochterski J., Petersson G. A., Ayala P. Y., Cui Q., Morokuma K., Rega N., Salvador P., Dannenberg J. J., Malick D. K., Rabuck A. D., Raghavachari K., Foresman J. B., Cioslowski J., Ortiz J.V., Baboul A. G., Stefanov B. B., Liu G., Liashenko A., Piskorz P., Komaromi I., Gomperts R., Martin R. L., Fox D. J., Keith T., Al-Laham M. A., Peng C. Y., Nanayakkara A., Challacombe M., Gill P. M. W., Johnson B., Chen W., Wong M. W., Andres J. L., Gonzalez C., Head-Gordon M., Replogle E.S., Pople J. A., Gaussian 03, Gaussian Inc., Pittsburgh, PA, 2003 |
| [36] | Boys S. F., Bernardi F., Mol. Phys., 1970, 19, 553—566 |
| [37] | Cioslowski J., Nanayakkara A., Challacombe M., Chem. Phys. Lett., 1993, 203, 137—142 |
| [38] | Cioslowski J., Surjan P. R., J. Mol. Struct.: Theochem., 1992, 255, 9—33 |
| [39] | Bader R. F. W., Encyclopedia of Computational Chemistry, 1998, 1, 64—86 |
| [40] | Bader R. F. W., Chem. Rev., 1991, 91, 893—928 |
| [1] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [2] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [3] | WANG Sijia, HOU Lu, LI Chenglong, LI Wencui, LU Anhui. Recent Advances in Synthesis and Applications of Hollow Nano-carbons [J]. Chem. J. Chinese Universities, 0, (): 20220637. |
| [4] | WU Qingying, ZHU Zhenyu, WU Jianming, XU Xin. A Dataset Representativeness Metric and A Slicing Sampling Strategy for the Kennard-Stone Algorithm [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220397. |
| [5] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [6] | ZHANG Lingyu, ZHANG Jilong, QU Zexing. Dynamics Study of Intramolecular Vibrational Energy Redistribution in RDX Molecule [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220393. |
| [7] | SHEN Qi, CHEN Haiyao, GAO Denghui, ZHAO Xi, NA Risong, LIU Jia, HUANG Xuri. A Study on the Interaction Mechanism of the Natural Product Falcarindiol with Human GABAA Receptor [J]. Chem. J. Chinese Universities, 0, (): 0. |
| [8] | CHEN Shaochen, CHENG Min, WANG Shihui, WU Jinkui, LUO Lei, XUE Xiaoyu, JI Xu, ZHANG Changchun, ZHOU Li. Transfer Learning Modeling for Predicting the Methane and Hydrogen Delivery Capacity of Metal-Organic Frameworks [J]. Chem. J. Chinese Universities, 0, (): 20220459. |
| [9] | PENG Xinzhe, GE Jiaoyang, WANG Fangli, YU Guojing, ZHOU Dong, RAN Xueqin, YANG Lei, XIE Linghai. A Theoretical Study on Tension and Reorganization Energy of Benzothiophene Grid [J]. Chem. J. Chinese Universities, 0, (): 20220313. |
| [10] | GUO Cheng, ZHANG Wei, TANG Yun. Ordered Mesoporous Materials: History, Progress and Perspective [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220167. |
| [11] | TANG Qiaowei, CAI Xiaoqing, LI Jiang, ZHU Ying, WANG Lihua, TIAN Yang, FAN Chunhai, HU Jun. Synchrotron-based X-ray Microscopy for Brain Imaging [J]. Chem. J. Chinese Universities, 0, (): 20220379. |
| [12] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [13] | DAI Wei, HOU Hua, WANG Baoshan. Theoretical Investigations on the Electronic Structures and Reactivity of Heptafluoro-iso-butyronitrile Anion [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220044. |
| [14] | SHI Naike, ZHANG Ya, SANSON Andrea, WANG Lei, CHEN Jun. Uniaxial Negative Thermal Expansion and Mechanism in Zn(NCN) [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220124. |
| [15] | REN Nana, XUE Jie, WANG Zhifan, YAO Xiaoxia, WANG Fan. Effects of Thermodynamic Data on Combustion Characters of 1,3-Butadiene [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220151. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||