

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (1): 59.doi: 10.7503/cjcu20150421
• Organic Chemistry • Previous Articles Next Articles
LÜ Haoting, MIAO Qun, SUN Huailin*( )
)
Received:2015-05-22
Online:2016-01-10
Published:2015-12-20
Contact:
SUN Huailin
E-mail:sunhl@nankai.edu.cn
Supported by:TrendMD:
LÜ Haoting, MIAO Qun, SUN Huailin. Alternating Copolymerization of Aryl Aldehyde Imines and Carbon Monoxide†[J]. Chem. J. Chinese Universities, 2016, 37(1): 59.
| Imine | Appearance | Yield(%) | b.p./℃ | IR(KBr), /cm-1 | HRMS(ESI), [M+H]+(cacld.), m/z |
|---|---|---|---|---|---|
| 1 | Colourless liquid | 88 | 54—56(66.7 Pa) | 1642 | 148.1125(148.1121) |
| 2 | Colourless liquid | 92 | 80—82(13.3 Pa) | 1650 | 192.1387(192.1383) |
| 3 | Colourless liquid | 94 | 56—58(533.3 Pa) | 1651 | 134.0968(134.0964) |
| 4 | Colourless liquid | 92 | 80—82(66.7 Pa) | 1651 | 164.1072(164.1070) |
| 5 | White solid | 98 | 56—58(13.3 Pa) | 1645 | 180.1021(180.1019) |
| 6 | White solid | 89 | 64—66(26.7 Pa) | 1637 | 164.1075(164.1070) |
Table 1 Appearance, yields, boiling points, IR data and HRMS data of imines 1—6
| Imine | Appearance | Yield(%) | b.p./℃ | IR(KBr), /cm-1 | HRMS(ESI), [M+H]+(cacld.), m/z |
|---|---|---|---|---|---|
| 1 | Colourless liquid | 88 | 54—56(66.7 Pa) | 1642 | 148.1125(148.1121) |
| 2 | Colourless liquid | 92 | 80—82(13.3 Pa) | 1650 | 192.1387(192.1383) |
| 3 | Colourless liquid | 94 | 56—58(533.3 Pa) | 1651 | 134.0968(134.0964) |
| 4 | Colourless liquid | 92 | 80—82(66.7 Pa) | 1651 | 164.1072(164.1070) |
| 5 | White solid | 98 | 56—58(13.3 Pa) | 1645 | 180.1021(180.1019) |
| 6 | White solid | 89 | 64—66(26.7 Pa) | 1637 | 164.1075(164.1070) |
| Entry | Imine | n(M)/n(C)b | t/h | Yield(%) | Polypeptide | 10-3 | PDId |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 10∶1 | 12 | 100 | 7 | 1.8 | |
| 2 | 1 | 20∶1 | 24 | 100 | 7 | 5.3 | 1.33 |
| 3 | 1 | 30∶1 | 30 | 100 | 7 | 6.8 | 1.43 |
| 4 | 2 | 10∶1 | 24 | 100 | 8 | 5.9 | 1.39 |
| 5 | 2 | 20∶1 | 24 | 45 | 8 | 7.4 | 1.43 |
| 6 | 3 | 10∶1 | 12 | 100 | 9 | 3.0 | 1.42 |
| 7 | 3 | 20∶1 | 24 | 43 | 9 | 2.9 | 1.49 |
| 8 | 4 | 10∶1 | 10 | 100 | 10 | 5.3 | 1.28 |
| 9 | 4 | 20∶1 | 24 | 60 | 10 | 5.3 | 1.67 |
| 10 | 5 | 10∶1 | 16 | 100 | 11 | 3.6 | 1.44 |
| 11 | 5 | 20∶1 | 24 | 37 | 11 | 3.2 | 1.35 |
| 12 | 6 | 20∶1 | 24 | 100 | 12 | 3.8 | 1.66 |
| 13 | 6 | 30∶1 | 36 | 52 | 12 | 5.1 | 1.69 |
Table 4 Copolymerization of imines and CO to produce polypeptidesa
| Entry | Imine | n(M)/n(C)b | t/h | Yield(%) | Polypeptide | 10-3 | PDId |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 10∶1 | 12 | 100 | 7 | 1.8 | |
| 2 | 1 | 20∶1 | 24 | 100 | 7 | 5.3 | 1.33 |
| 3 | 1 | 30∶1 | 30 | 100 | 7 | 6.8 | 1.43 |
| 4 | 2 | 10∶1 | 24 | 100 | 8 | 5.9 | 1.39 |
| 5 | 2 | 20∶1 | 24 | 45 | 8 | 7.4 | 1.43 |
| 6 | 3 | 10∶1 | 12 | 100 | 9 | 3.0 | 1.42 |
| 7 | 3 | 20∶1 | 24 | 43 | 9 | 2.9 | 1.49 |
| 8 | 4 | 10∶1 | 10 | 100 | 10 | 5.3 | 1.28 |
| 9 | 4 | 20∶1 | 24 | 60 | 10 | 5.3 | 1.67 |
| 10 | 5 | 10∶1 | 16 | 100 | 11 | 3.6 | 1.44 |
| 11 | 5 | 20∶1 | 24 | 37 | 11 | 3.2 | 1.35 |
| 12 | 6 | 20∶1 | 24 | 100 | 12 | 3.8 | 1.66 |
| 13 | 6 | 30∶1 | 36 | 52 | 12 | 5.1 | 1.69 |
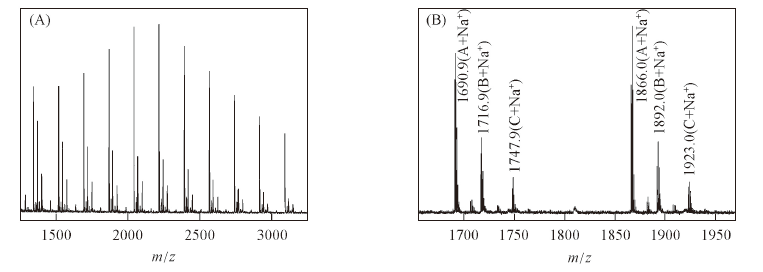
Fig.2 MALDI-TOF MS spectra of polypeptide 7(Entry 3 in Table 4)^(A) Full spectrum with n values given for individual peaks; (B) expansion of the m/z region from 1650 to 1960, in which the m/z difference between peaks of (A+Na+) with n=9 and n=10 is 175.1.
| [1] | Bodanszky M., Bodanszky A., Principles of Peptide Synthesis, 2nd Ed., Springer, New York, 1994, 215—318 |
| [2] | Sewald N., Jakubke H.D., Peptides: Chemistry and Biology, Wiley-VCH, Weinheim, 2002, 61—130 |
| [3] | Luxenhofer R., Fetsch C., Grossmann A., J. Polym. Sci. Pol. Chem., 2013, 51, 2731—2752 |
| [4] | Sun J., Zuckermann R. N., ACS Nano, 2013, 7, 4715—4732 |
| [5] | Xin J., Zhang Y., Zhang L., Lin Y., Zhou Q., Chem. Res. Chinese Universities, 2013, 29(5), 924—928 |
| [6] | Fetsch C., Grossmann A., Holz L., Nawroth J. F., Luxenhofer R., Macromolecules,2011, 44, 6746—6758 |
| [7] | Kricheldorf H. R., Angew. Chem. Int. Ed., 2006, 45, 5752—5784 |
| [8] | Pattabiraman V. R., Bode J. W., Nature,2011, 480, 471—479 |
| [9] | Deming T. J., Adv. Mater., 1997, 9, 299—311 |
| [10] | Sen A., Acc. Chem. Res., 1993, 26, 303—310 |
| [11] | Drent E., Budzelaar P. H. M., Chem. Rev., 1996, 96, 663—681 |
| [12] | Kacker S., Kim J. S., Sen A., Angew. Chem. Int. Ed., 1998, 37, 1251—1253 |
| [13] | Dghaym R. D., Yaccato K. J., Arndtsen B. A., Organomeallics,1998, 17, 4—6 |
| [14] | Cavallo L., J. Am. Chem. Soc., 1999, 121, 4238—4241 |
| [15] | Davis J. L., Arndtsen B. A., Organometallics,2000, 19, 4657—4659 |
| [16] | Lafrance D. , Davis J. L., Dhawan R., Arndtsen B. A., Organometallics,2001, 20, 1128—1136 |
| [17] | Sun H., Zhang J., Liu Q., Zhao J., Yu L., Angew. Chem. Int. Ed., 2007, 46, 6068—6072 |
| [18] | Zhang Y. P., Sun H. L., Chem. J. Chinese Universities, 2014, 35(1), 54—57 |
| (张永坡, 孙怀林. 高等学校化学学报, 2014, 35(1), 54—57) | |
| [19] | Jia L., Sun H., Shay J. T., Allgeier A. M., Hanton S. D., J. Am. Chem. Soc., 2002, 124, 7282—7283 |
| [20] | Yoshida H., Fukushima H., Ohshita J., Kunai A., J. Am. Chem. Soc., 2006, 128, 11040—11041 |
| [21] | Karabatsos G. J., Lande S. S., Tetrahedron,1968, 24, 3907—3922 |
| [22] | Joly G. D., Jacobsen E. N., J. Am. Chem. Soc., 2004, 126, 4102—4103 |
| [23] | Baldridge A., Kowalik J., Tolbert L. M., Synthesis,2010, 14, 2424—2436 |
| [24] | Yudin L. G., Zh. Org. Khim., 1983, 11, 2361—2366 |
| [25] | Moffett R. B., Hoehn W. M., J. Am. Chem. Soc., 1947, 69, 1792—1794 |
| [26] | Dghaym R. D., Dhawan R., Arndtsen B. A., Angew. Chem. Int. Ed., 2001, 40, 3228—3230 |
| [27] | Speckampa W. N., Hiemstra H., Tetrahedron,1985, 41, 4367—4416 |
| [1] | TANG Quanjun, LIU Yingxin, MENG Rongwei, ZHANG Ruotian, LING Guowei, ZHANG Chen. Application of Single-atom Catalysis in Marine Energy [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220324. |
| [2] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [3] | YAO Qing, YU Zhiyong, HUANG Xiaoqing. Progress in Synthesis and Energy-related Electrocatalysis of Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220323. |
| [4] | LIN Zhi, PENG Zhiming, HE Weiqing, SHEN Shaohua. Single-atom and Cluster Photocatalysis: Competition and Cooperation [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220312. |
| [5] | TENG Zhenyuan, ZHANG Qitao, SU Chenliang. Charge Separation and Surface Reaction Mechanisms for Polymeric Single-atom Photocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220325. |
| [6] | YANG Jingyi, SHI Siqi, PENG Huaitao, YANG Qihao, CHEN Liang. Integration of Atomically Dispersed Ga Sites with C3N4 Nanosheets for Efficient Photo-driven CO2 Cycloaddition [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220349. |
| [7] | WANG Ruyue, WEI Hehe, HUANG Kai, WU Hui. Freezing Synthesis for Single Atom Materials [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220428. |
| [8] | WANG Xintian, LI Pan, CAO Yue, HONG Wenhao, GENG Zhongxuan, AN Zhiyang, WANG Haoyu, WANG Hua, SUN Bin, ZHU Wenlei, ZHOU Yang. Techno-economic Analysis and Industrial Application Prospects of Single-atom Materials in CO2 Catalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220347. |
| [9] | YANG Jingyi, LI Qinghe, QIAO Botao. Synergistic Catalysis Between Ir Single Atoms and Nanoparticles for N2O Decomposition [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220388. |
| [10] | LIN Gaoxin, WANG Jiacheng. Progress and Perspective on Molybdenum Disulfide with Single-atom Doping Toward Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220321. |
| [11] | WANG Sicong, PANG Beibei, LIU Xiaokang, DING Tao, YAO Tao. Application of XAFS Technique in Single-atom Electrocatalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220487. |
| [12] | HAN Fuchao, LI Fujin, CHEN Liang, HE Leiyi, JIANG Yunan, XU Shoudong, ZHANG Ding, QI Lu. Enhance of CoSe2/C Composites Modified Separator on Electrochemical Performance of Li-S Batteries at High Sulfur Loading [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220163. |
| [13] | HUANG Qiuhong, LI Wenjun, LI Xin. Organocatalytic Enantioselective Mannich-type Addition of 5H-Oxazol-4-ones to Isatin Derived Ketimines [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220131. |
| [14] | TAN Yan, YU Shen, LYU Jiamin, LIU Zhan, SUN Minghui, CHEN Lihua, SU Baolian. Efficient Preparation of Mesoporous γ-Al2O3 Microspheres and Performance of Pd-loaded Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220133. |
| [15] | XIA Wu, REN Yingyi, LIU Jing, WANG Feng. Chitosan Encapsulated CdSe QDs Assemblies for Visible Light-induced CO2 Reduction in an Aqueous Solution [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220192. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||