

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (10): 2055.doi: 10.7503/cjcu20140555
• Articles: Inorganic Chemistry • Previous Articles Next Articles
WANG Shuhua*( ), HU Hanzhen, CHEN Chao, MA Running, ZHANG Ning*(
), HU Hanzhen, CHEN Chao, MA Running, ZHANG Ning*( )
)
Received:2014-06-20
Online:2014-10-10
Published:2014-09-30
Contact:
WANG Shuhua,ZHANG Ning
E-mail:shwang@ncu.edu.cn;nzhang. ncu@163.com
Supported by:CLC Number:
TrendMD:
WANG Shuhua, HU Hanzhen, CHEN Chao, MA Running, ZHANG Ning. Lanthanide Metal-organic Frameworks Constructed from Evolution of a C3 Symmetry Ligand: Syntheses,Structures and Luminescent Properties†[J]. Chem. J. Chinese Universities, 2014, 35(10): 2055.
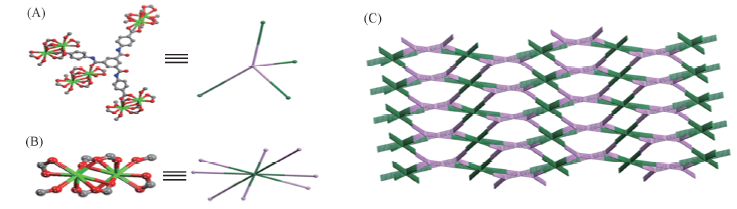
Fig.3 Ball and stick schematic representations of 4-connected ligand(pink, A) and 8-connected Pr dimmer(green, B) and schematic representation of (4,8)-connected AlB2 topology(C) of compound 3
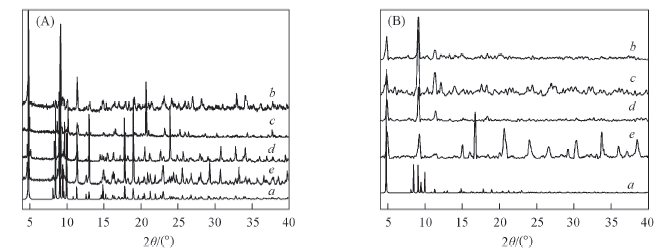
Fig.4 Simulated XRD pattern of compound 3(a) and experimental PXRD patterns of compounds 1—4(b—e, respectively)(A) and compound 3 after capturing/adsorbing Eosin(b), La3+(c), Cr3+(d) and methanol(e)(B)
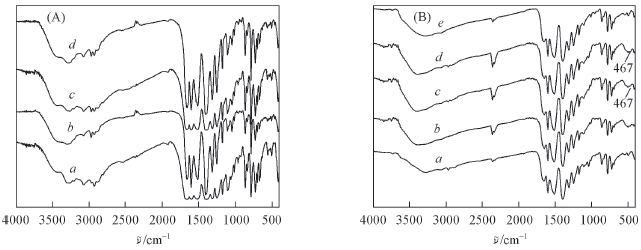
Fig.5 FTIR spectra of compounds 1—4(a—d, respectively)(A) and compound 3 before(a) and after capturing/adsorbing methanol(b), Cr3+(c), La3+(d) and Cu2+(e)(B)
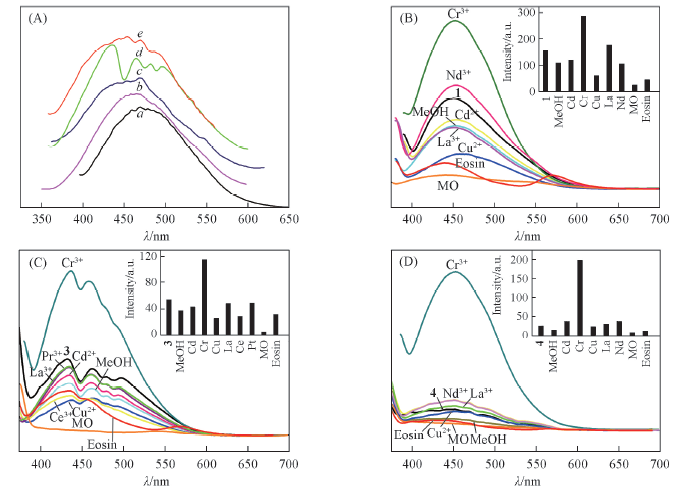
Fig.7 Fluorescence emission spectra of ligand BTMC(a) and compounds 1—4(b—e, respectively)(A) and fluorenscence spectra of compounds 1(B), 3(C) and 4(D) with different ions, methyl orange(MO) and Eosin, respectively The inset bar charts represent the intensity changes of the emission with different ions and dyes.
| [1] | Furukawa H., Cordova K. E., O’Keeffe M., Yaghi O. M., Science,2013, 341, 974—987 |
| [2] | Furukawa H., Ko N., Go Y. B., Aratani N., Choi S. B., Choi E., Yazaydin A., Snurr R., O’Keeffe M., Kim J., Yaghi O. M., Science,2010, 329, 424—428 |
| [3] | Yuan D., Zhao D., Sun D., Zhou H. C., Angew. Chem. Int. Ed., 2010, 49, 5357—5361 |
| [4] | Yang J., Ma J. F., Liu Y. Y., Li S. L., Zheng G. L., Eur. J. Inorg. Chem., 2005, 2005(11), 2174—2180 |
| [5] | Yang J., Ma J. F., Liu Y. Y., Ma J. C., Jia H. Q., Hu N. H., Eur. J. Inorg. Chem., 2006, 2006(6), 1208—1215 |
| [6] | Ma R., Chen C., Sun B. Zhao X., Zhang N., Inorg. Chem. Commun., 2011, 14, 1532—1536 |
| [7] | Spek A. L., J. Appl. Crystallogr., 2003, 36, 7—13 |
| [8] | Baburin I. A., Blatov V. A., Carluccib L., Ciani G., Proserpio D. M., J. Solid State Chem., 2005, 178, 2452—2474 |
| [9] | Chen C., Wang S. H., Zhang N., Yan Z. H., Pang W. Q., Micropor. Mesopor. Mater., 2007, 106, 1—7 |
| [10] | Chandra S. K., Basu P., Ray D., Pal S., Chakravorty A., Inorg. Chem., 1990, 29, 2423—2428 |
| [11] | Allendorf M. D., Bauer C. A., Bhakta R. K., Houk R. J. T., Chem. Soc. Rev., 2009, 38, 1330—1352 |
| [12] | Liang L. L., Ren S. B., Zhang J., Li Y. Z., Du H. B., You X. Z., Cryst. Growth Des., 2010, 10, 1307—1311 |
| [13] | Hou M., Zhang J., J. Anal. Sci., 2004, 20, 619—621 |
| (侯明, 张静. 分析科学学报, 2004, 20, 619—621) | |
| [14] | Xu L., Chen C., Wang R., Luo J. H., Liu Y. L., Zhang N., Chem. J. Chinese Universities,2013, 34(8), 1907—1912 |
| (徐力, 陈超, 王瑞, 罗家还, 刘云凌, 张宁. 高等学校化学学报, 2013, 34(8), 1907—1912) | |
| [15] | Li Y. X., Xue M., Guo L. J., Huang L., Chen S. R., Qiu S. L., Chem. Res. Chinese Universities,2013, 29(2), 196—200 |
| [16] | Cui Y. J., Yue Y. F., Qian G. D., Chen B. L., Chem. Rev., 2012, 112, 1126—1162 |
| [17] | Arduini A., Giorgi G., Pochini A., Secchi A., Ugozzoli F., Tetrahedron,2001, 57, 2411—2417 |
| [1] | HAN Zongsu, YU Xiaoyong, MIN Hui, SHI Wei, CHENG Peng. A Rare Earth Metal-Organic Framework with H6TTAB Ligand [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210342. |
| [2] | LIU Xueguang, YANG Xiaoshan, MA Jingjing, LIU Weisheng. Separating Methyl Blue Selectively from the Mixture of Dyes by Europium Metal-organic Frameworks [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210715. |
| [3] | ZHAO Yuxuan,CHEN Yanjun,PAN Guxin,WANG Chang,PENG Zhenbo,SUN Zongxu,LIANG Yongri,SHI Qisong. Preparation and Performance of Novel Tb-PEG+Eu-PEG/PANI/PAN Luminescent-electrical-phase Change Composite Fibers by Electrospinning† [J]. Chem. J. Chinese Universities, 2019, 40(4): 824. |
| [4] | FU Linjie,LUO Ran,WANG Shuhua,ZHANG Ning,CHEN Chao. Synthesis of Novel Cd(Ⅱ) Metal-organic Framework for Highly Selective Detection of p-Nitroaniline† [J]. Chem. J. Chinese Universities, 2019, 40(3): 419. |
| [5] | ZHANG Chunyan,LUO Jianxin,LI Wenjun,OU Lijuan,YU Guipeng,PAN Chunyue. Preparation and Sensing Properties of Covalent-linked Europium Complex Monodisperse Polystyrene Microspheres† [J]. Chem. J. Chinese Universities, 2019, 40(1): 153. |
| [6] | WANG Caiping, LI Youfen, ZHANG Yidong. Preparation of Glass Ceramics Containing CaF2 by One-step Method and Analysis of the Fluorescent Probe of Eu3+ Ion† [J]. Chem. J. Chinese Universities, 2016, 37(4): 607. |
| [7] | KANG Xiaoyan, HE Anqi, WANG Jingdan, GUO Ran, ZHAI Yanjun, XU Yizhuang, WU Jinguang. Investigation on the Enrichment of DNA Using Lanthanum Carbonate† [J]. Chem. J. Chinese Universities, 2016, 37(1): 7. |
| [8] | LI Xu, JIANG Jianhong, HAN Buxing, GU Huiwen, XIE Zhaofeng, CHEN Lan, XIAO Shengxiong, LI Chuanhua, LI Aitao, LI Xia, YAO Feihong, WANG Qun, LI Qiangguo. Synthesis and Biological Activities of o-Vanillin-histidine Schiff-base and Its Lanthanum Complex† [J]. Chem. J. Chinese Universities, 2015, 36(5): 856. |
| [9] | LIN Xiaomin, ZHU Lili, HAN Jian, LIU Xiaomei. Microstructure and Electrical Properties of Solid Electrolytes Ce0.9Er0.1-xPrx [J]. Chem. J. Chinese Universities, 2015, 36(1): 61. |
| [10] | WANG Shuhua, WANG Pingping, LI Pengfei, ZHANG Ning, CHEN Chao. Postsynthetic Modification of UMCM-1-NH2 and Fluorescence Recognition for Magnesium† [J]. Chem. J. Chinese Universities, 2014, 35(12): 2499. |
| [11] | CHI Yuxian, QIU Juqing, LI Shumei, YANG Yanhong, JIN Jing, NIU Shuyun. Syntheses, Structures and Photophysical Properties of a Series of Sm3+ Coordination Complexes† [J]. Chem. J. Chinese Universities, 2014, 35(8): 1620. |
| [12] | ZHAO Binyu, LIANG Xiaojuan, CHEN Zhaoping, XIE Cuiping, LUO Le, ZHANG Zhimin, ZHONG Jiasong, XIANG Weidong. Studies on Optical Properties and Ce Concentration of Ce∶YAG Single Crystal for WLEDs† [J]. Chem. J. Chinese Universities, 2014, 35(2): 230. |
| [13] | LIU Yang, JI Hongwei, ZHOU Defeng, ZHU Xiaofei, LI Zhaohui. Controllable Synthesis and Photocatalytic Activity of TiO2/LaFeO3 Micro-nanofibers† [J]. Chem. J. Chinese Universities, 2014, 35(1): 19. |
| [14] | CHEN Ying, LI Fei-Fei, LI Chun-Guang, PAN Qing-Zhi, CUI Man-Hua. Preparation of Up-conversion Luminescent Material Labeled Antibody and Its Application in Immunohistochemistry [J]. Chem. J. Chinese Universities, 2013, 34(4): 788. |
| [15] | JIANG Ying-Ying, LIU Gui-Xia, WANG Jin-Xian, DONG Xiang-Ting, YU Wen-Sheng. Hydrothermal Preparation and Luminescence Properties of NaGd(MoO4)2: Dy3+,Eu3+ White-light Phosphors [J]. Chem. J. Chinese Universities, 2013, 34(4): 794. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||