

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (12): 2654.doi: 10.7503/cjcu20140464
• Physical Chemistry • Previous Articles Next Articles
LI Yanbin1,2, XU Ying2,3, MA Longlong1,2,3,*( ), ZHANG Qi2,3, WANG Tiejun2,3, CHEN Guanyi1, ZHANG Limin2
), ZHANG Qi2,3, WANG Tiejun2,3, CHEN Guanyi1, ZHANG Limin2
Received:2014-05-19
Online:2014-12-10
Published:2014-11-29
Contact:
MA Longlong
E-mail:mall@ms.giec.ac.cn
Supported by:CLC Number:
TrendMD:
LI Yanbin, XU Ying, MA Longlong, ZHANG Qi, WANG Tiejun, CHEN Guanyi, ZHANG Limin. Research on in situ Hydrogenation of o-Cresol over Ni/CMK-3 Catalysts†[J]. Chem. J. Chinese Universities, 2014, 35(12): 2654.
| Catalyst | Specific surface area/(m2·g-1) | Average pore size/nm | Pore volume/(cm3·g-1) |
|---|---|---|---|
| CMK-3 | 1128.026 | 3.411 | 0.781 |
| 20%Ni/CMK-3 | 984.706 | 3.399 | 0.752 |
| SBA-15 | 674.972 | 6.419 | 0.907 |
| 20%Ni/SBA-15 | 558.942 | 6.581 | 0.964 |
Table 1 Textural and structural properties of the catalysts
| Catalyst | Specific surface area/(m2·g-1) | Average pore size/nm | Pore volume/(cm3·g-1) |
|---|---|---|---|
| CMK-3 | 1128.026 | 3.411 | 0.781 |
| 20%Ni/CMK-3 | 984.706 | 3.399 | 0.752 |
| SBA-15 | 674.972 | 6.419 | 0.907 |
| 20%Ni/SBA-15 | 558.942 | 6.581 | 0.964 |
| Reaction time/h | Conversion of methanol(%) | Selectivity of methane(%) | Reaction time/h | Conversion of methanol(%) | Selectivity of methane(%) |
|---|---|---|---|---|---|
| 3 | 5.41 | 3.19 | 9 | 11.21 | 7.06 |
| 6 | 7.47 | 4.57 | 12 | 12.45 | 9.52 |
Table 2 Catalytic effect contrast of Ni/CMK-3 catalsts
| Reaction time/h | Conversion of methanol(%) | Selectivity of methane(%) | Reaction time/h | Conversion of methanol(%) | Selectivity of methane(%) |
|---|---|---|---|---|---|
| 3 | 5.41 | 3.19 | 9 | 11.21 | 7.06 |
| 6 | 7.47 | 4.57 | 12 | 12.45 | 9.52 |
| Hydrogen donor solvent | Conversion of o-cresol(%) | Selectivity of product(%) | Real hydrogen yield/mol | |
|---|---|---|---|---|
| 2-Methyl cyclohexanol | 2-Methyl cyclohexanone | |||
| Isopropyl alcohol | 26.72 | 15.63 | 83.22 | 0.008 |
| Methanol | 53.49 | 36.73 | 63.20 | 0.031 |
| Glycerol | 64.41 | 32.14 | 36.81 | 0.026 |
| Formic acid | 82.24 | 39.45 | 60.48 | 0.041 |
Table 3 Effects of hydrogen-donor solvents on catalystic performance
| Hydrogen donor solvent | Conversion of o-cresol(%) | Selectivity of product(%) | Real hydrogen yield/mol | |
|---|---|---|---|---|
| 2-Methyl cyclohexanol | 2-Methyl cyclohexanone | |||
| Isopropyl alcohol | 26.72 | 15.63 | 83.22 | 0.008 |
| Methanol | 53.49 | 36.73 | 63.20 | 0.031 |
| Glycerol | 64.41 | 32.14 | 36.81 | 0.026 |
| Formic acid | 82.24 | 39.45 | 60.48 | 0.041 |
| Reactant | Conversion of reactant(%) | Selectivity of product(%) | Conversion of methanol(%) | |
|---|---|---|---|---|
| 2-Methylcyclohexanol | 2-Methylcyclohexanone | |||
| o-Cresol | 45.03 | 36.73 | 63.20 | 10.34 |
| m-Cresol | 43.79 | 39.45 | 60.48 | 11.09 |
| p-Cresol | 27.62 | 33.48 | 65.71 | 11.62 |
Table 4 Comparsion of the effect on three isomers
| Reactant | Conversion of reactant(%) | Selectivity of product(%) | Conversion of methanol(%) | |
|---|---|---|---|---|
| 2-Methylcyclohexanol | 2-Methylcyclohexanone | |||
| o-Cresol | 45.03 | 36.73 | 63.20 | 10.34 |
| m-Cresol | 43.79 | 39.45 | 60.48 | 11.09 |
| p-Cresol | 27.62 | 33.48 | 65.71 | 11.62 |
| Reaction times | Conversion of o-cresol(%) | Selectivity of product(%) | Conversion of methanol(%) | |
|---|---|---|---|---|
| 2-Methyl cyclohexanol | 2-Methyl cyclohexanone | |||
| 1 | 45.03 | 36.73 | 63.20 | 10.34 |
| 2 | 40.79 | 34.17 | 65.71 | 8.41 |
| 3 | 25.86 | 39.45 | 60.48 | 2.48 |
| 4 | 6.82 | 11.37 | 88.44 | 0.92 |
Table 5 Testing effect of CMK-3 reaction life
| Reaction times | Conversion of o-cresol(%) | Selectivity of product(%) | Conversion of methanol(%) | |
|---|---|---|---|---|
| 2-Methyl cyclohexanol | 2-Methyl cyclohexanone | |||
| 1 | 45.03 | 36.73 | 63.20 | 10.34 |
| 2 | 40.79 | 34.17 | 65.71 | 8.41 |
| 3 | 25.86 | 39.45 | 60.48 | 2.48 |
| 4 | 6.82 | 11.37 | 88.44 | 0.92 |
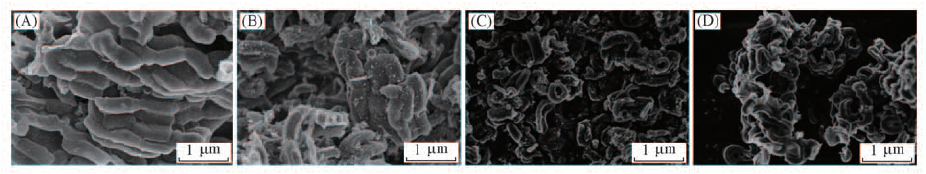
Fig.11 SEM images of the catalysts after reaction (A) After first reaction; (B) after second reaction; (C) after third reaction; (D) after fourth reaction.
| [1] | Elliott D. C., Energy Fuels, 2007, 21(3), 1792—1815 |
| [2] | Fisk C. A., Morgan T., Ji Y., Crocker M., Crofcheck C., Lewis S. A., Appl. Catal. A, 2009, 358(2), 150—156 |
| [3] | Graça I., Lopes J. M., Cerqueira H. S., Ribeiro M. F., Ind. Eng. Chem. Res., 2012, 121224065214005 |
| [4] | Donnis B., Egeberg R. G., Blom P., Knudsen K. G., Top. Catal., 2009, 52(3), 229—240 |
| [5] | French R. J., Stunkel J., Baldwin R. M., Energy Fuels, 2011, 25(7), 3266—3274 |
| [6] | Oasmaa A., Kuoppala E., Elliott D. C., Energy Fuels, 2012, 26(4), 2454—2460 |
| [7] | Şenol O.İ., Ryymin E. M., Viljava T. R., Krause A. O. I., J. Mol. Catal. A: Chem., 2007, 277(1/2), 107—112 |
| [8] | Furimsky E., Appl. Catal. A: Gene., 2000, 199(2), 147—190 |
| [9] | Moraes M. S. A., Migliorini M. V., Damasceno F. C., Georges F., Almeida S., Zini C. A., Jacques R. A., Caramao E. B., J. Anal. Appl. Pyrolysis, 2012, 98, 51—64 |
| [10] | Guo J., Ruan R., Zhang Y., Ind. Eng. Chem. Res., 2012, 51(19), 6599—6604 |
| [11] | Wu C., Liu R., Energy Fuels, 2010, 24(9), 5139—5147 |
| [12] | Zhao H. Y., Li D., Bui P., Oyama S. T., Appl. Catal. A: Gen., 2011, 391(1/2), 305—310 |
| [13] | Echeandia S., Arias P. L., Barrio V. L., Pawelec B., Fierro J. L. G., Appl. Catal. B, 2010, 101(1/2), 1—12 |
| [14] | Wang S. R., Yin Q. Q., Li X. B., Chem. Res. Chinese Universities, 2012, 28(1), 119—123 |
| [15] | Yan S. W., Fan H., Liang C., Li Z., Chem. J. Chinese Universities, 2012, 33(9), 2067—2073 |
| (闫少伟, 范辉, 梁川, 李忠. 高等学校化学学报, 2012, 33(9), 2067—2073) | |
| [16] | Xiang Y., Kong L., Xie P., Xu T., Wang J., Li X., Ind. Eng. Chem. Res., 2014, 53(6), 2197—2203 |
| [17] | Xiang Y., Li X., Lu C., Ma L., Yuan J., Feng F., Ind. Eng. Chem. Res., 2011, 50(6), 3139—3144 |
| [18] | Li X. N., Xiang Y. Z., Science in China, Series B,2007, 37(2), 136—142 |
| (李小年, 项益智. 中国科学, B辑,2007, 37(2), 136—142) | |
| [19] | Wei S., Cui H., Wang J., Zhuo S., Yi W., Wang L., Li Z., Particuology,2011, 9(1), 69—74 |
| [20] | He X., Ling P., Qiu J., Yu M., Zhang X., Yu C., Zheng M., J. Power Sources, 2013, 240, 109—113 |
| [21] | Tan Z., Xu X., Liu Y., Zhang C., Zhai Y., Liu P., Li Y., Zhang R., Environmental Progress & Sustainable Energy,2014, 33(3), 751—755 |
| [22] | Jun S., Joo S. H., Ryoo R., Kruk M., Jaroniec M., Liu Z., Ohsuna T., Terasaki O., J. Am. Chem. Soc., 2000, 122(43), 10712—10713 |
| [23] | Cao Y. L., Cao J. M., Zheng M. B., Liu J. S., Ji G. B., J. Solid State Chem., 2007, 180(2), 792—798 |
| [24] | Chen T., Li W. Z., Yu C. Y., Acta Phys. Chim. Sin., 1999, (7), 613—618 |
| (陈铜, 李文钊, 于春英. 物理化学学报, 1999, (7), 613—618) | |
| [25] | Lin X. P., Wu G. Y., Zhou Y. S., Feng Y. G., J. Jiangsu Polytechnic University, 2005, (4), 1—5 |
| (林西平, 邬国英, 周永生, 冯艳果. 江苏工业学院学报, 2005, (4), 1—5) | |
| [26] | Ma T. Y., Liu L., Yuan Z. Y., Chem. Soc. Rev., 2013, 42(9), 3977—4003 |
| [27] | Jiang T., Chen S. S., Cao F. H., Chem. Ind. Eng. Prog., 2012, (5), 1010—1017 |
| (江涛, 陈诗诗, 曹发海. 化工进展, 2012, (5), 1010—1017) | |
| [28] | Yu Y. X., Xu Y., Wang T. J., Ma L. L., Zhang Q., Zhang X. H., Zhang X., Journal of Fuel Chemistry and Technology, 2013, (4), 443—448 |
| [29] | Horáček J., Št'ávová G., Kelbichová V., Kubička D., Catal. Today, 2013, 204, 38—45 |
| [30] | Ausavasukhi A., Huang Y., To A. T., Sooknoi T., Resasco D. E., J. Catal., 2012, 290, 90—100 |
| [31] | Zanuttini M. S., Lago C. D., Querini C. A., Peralta M. A., Catal. Today, 2013, 213, 9—17 |
| [32] | Wan H., Chaudhari R. V., Subramaniam B., Top. Catal., 2012, 55(3/4), 129—139 |
| [33] | Wan H., Chaudhari R. V., Subramaniam B., Energy Fuels, 2013, 27(1), 487—493 |
| [1] | DONG Yanhong, LU Xinhuan, YANG Lu, SUN Fanqi, DUAN Jingui, GUO Haotian, ZHANG Qinjun, ZHOU Dan, XIA Qinghua. Preparation of Bifunctional Metal-organic Framework Materials and Application in Catalytic Olefins Epoxidation [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220458. |
| [2] | WANG Zumin, MENG Cheng, YU Ranbo. Doping Regulation in Transition Metal Phosphides for Hydrogen Evolution Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220544. |
| [3] | SONG Jiaxin, CUI Jing, FAN Xiaoqiang, KONG Lian, XIAO Xia, XIE Zean, ZHAO Zhen. Preparation of mesoporous silica supported highly dispersed vanadium catalyst and their catalytic performance for selective oxidation of ethane [J]. Chem. J. Chinese Universities, 0, (): 20220532. |
| [4] | TENG Zhenyuan, ZHANG Qitao, SU Chenliang. Charge Separation and Surface Reaction Mechanisms for Polymeric Single-atom Photocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220325. |
| [5] | LIN Gaoxin, WANG Jiacheng. Progress and Perspective on Molybdenum Disulfide with Single-atom Doping Toward Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220321. |
| [6] | LIU Shuanghong, XIA Siyu, LIU Shiqi, LI Min, SUN Jiajie, ZHONG Yong, ZHANG Feng, BAI Feng. Current Advances of Hollow All-solid-state Z-scheme Photocatalysts [J]. Chem. J. Chinese Universities, 0, (): 20220512. |
| [7] | ZHAO Yingzhe, ZHANG Jianling. Applications of Metal-organic Framework-based Material in Carbon Dioxide Photocatalytic Conversion [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220223. |
| [8] | DING Yang, WANG Wanhui, BAO Ming. Recent Progress in Porous Framework-immobilized Molecular Catalysts for CO2 Hydrogenation to Formic Acid [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220309. |
| [9] | SONG Youwei, AN Jiangwei, WANG Zheng, WANG Xuhui, QUAN Yanhong, REN Jun, ZHAO Jinxian. Effects of Ag,Zn,Pd-doping on Catalytic Performance of Copper Catalyst for Selective Hydrogenation of Dimethyl Oxalate [J]. Chem. J. Chinese Universities, 2022, 43(6): 20210842. |
| [10] | GAO Wenxiu, LYU Jieqiong, GAO Yongping, KONG Changjian, WANG Xueping, GUO Shengnan, LOU Dawei. Preparation of Ethyl α⁃Cyanocinnamate Catalyzed by Nitrogen-rich Porous Organic Polymers [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220078. |
| [11] | WANG Guangqi, BI Yiyang, WANG Jiabo, SHI Hongfei, LIU Qun, ZHANG Yu. Heterostructure Construction of Noble-metal-free Ternary Composite Ni(PO3)2-Ni2P/CdS NPs and Its Visible Light Efficient Catalytic Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220050. |
| [12] | WANG Hong, SAN Khin Nyein Ei, FANG Yun, ZHANG Xinyu, FAN Ye. Pickering Emulsion Stabilization and Interfacial Catalytic Oxidation by Janus Nano-Au [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220105. |
| [13] | HU Huimin, CUI Jing, LIU Dandan, SONG Jiaxin, ZHANG Ning, FAN Xiaoqiang, ZHAO Zhen, KONG Lian, XIAO Xia, XIE Zean. Influence of Different Transition Metal Decoration on the Propane Dehydrogenation Performance over Pt/M-DMSN Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210815. |
| [14] | ZHAO Wanjun, LI Xiao, Dang Hui, WANG Yongzhao, ZHAO Yongxiang. Preparation of Supported Pd-Cu Catalyst and Its Preferential Oxidation of CO Under Hydrogen-rich Atmosphere [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210754. |
| [15] | ZHOU Ning, TANG Xiaohua, CAO Hong, ZHA Fei, LI Chun, XIE Chunyan, XU Mingping, SUN Yige. Preparation, Characterization and Degradation to BPA of Pomegranate-like Gel Microsphere Entrapmented Laccase [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210705. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||